A synthetic synaptic organizer protein restores glutamatergic neuronal circuits
- PMID: 32855309
- PMCID: PMC7116145
- DOI: 10.1126/science.abb4853
A synthetic synaptic organizer protein restores glutamatergic neuronal circuits
Abstract
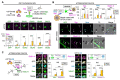
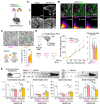
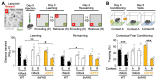
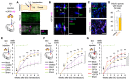
Neuronal synapses undergo structural and functional changes throughout life, which are essential for nervous system physiology. However, these changes may also perturb the excitatory-inhibitory neurotransmission balance and trigger neuropsychiatric and neurological disorders. Molecular tools to restore this balance are highly desirable. Here, we designed and characterized CPTX, a synthetic synaptic organizer combining structural elements from cerebellin-1 and neuronal pentraxin-1. CPTX can interact with presynaptic neurexins and postsynaptic AMPA-type ionotropic glutamate receptors and induced the formation of excitatory synapses both in vitro and in vivo. CPTX restored synaptic functions, motor coordination, spatial and contextual memories, and locomotion in mouse models for cerebellar ataxia, Alzheimer's disease, and spinal cord injury, respectively. Thus, CPTX represents a prototype for structure-guided biologics that can efficiently repair or remodel neuronal circuits.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures


Comment in
-
Restoring neuron connections.Science. 2020 Aug 28;369(6507):1052-1053. doi: 10.1126/science.abd4762. Science. 2020. PMID: 32855323 No abstract available.
-
Building synapses: Using a synthetic approach to bridge synaptic membranes.Fac Rev. 2022 Sep 21;11:25. doi: 10.12703/r-01-0000017. eCollection 2022. Fac Rev. 2022. PMID: 36262561 Free PMC article.
References
-
- Bozzi Y, Provenzano G, Casarosa S. Neurobiological bases of autism-epilepsy comorbidity: a focus on excitation/inhibition imbalance. Eur J Neurosci. 2018;47:534–548. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

