Senolytics prevent mt-DNA-induced inflammation and promote the survival of aged organs following transplantation
- PMID: 32855397
- PMCID: PMC7453018
- DOI: 10.1038/s41467-020-18039-x
Senolytics prevent mt-DNA-induced inflammation and promote the survival of aged organs following transplantation
Abstract
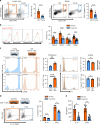
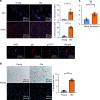
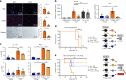
Older organs represent an untapped potential to close the gap between demand and supply in organ transplantation but are associated with age-specific responses to injury and increased immunogenicity, thereby aggravating transplant outcomes. Here we show that cell-free mitochondrial DNA (cf-mt-DNA) released by senescent cells accumulates with aging and augments immunogenicity. Ischemia reperfusion injury induces a systemic increase of cf-mt-DNA that promotes dendritic cell-mediated, age-specific inflammatory responses. Comparable events are observed clinically, with the levels of cf-mt-DNA elevated in older deceased organ donors, and with the isolated cf-mt-DNA capable of activating human dendritic cells. In experimental models, treatment of old donor animals with senolytics clear senescent cells and diminish cf-mt-DNA release, thereby dampening age-specific immune responses and prolonging the survival of old cardiac allografts comparable to young donor organs. Collectively, we identify accumulating cf-mt-DNA as a key factor in inflamm-aging and present senolytics as a potential approach to improve transplant outcomes and availability.
Conflict of interest statement
J.L.K. and T.T. have a financial interest related to this research. Patents on senolytic drugs are held by Mayo Clinic. This research has been reviewed by the Mayo Clinic Conflict of Interest Review Board and was conducted in compliance with Mayo Clinic Conflict of Interest policies. No conflicts of interest, financial or otherwise, are declared by the other authors.
Figures



Comment in
-
Transplantation From Older Donors: Can Senolytics Turn Back the Clock?Transplantation. 2021 Apr 1;105(4):681-682. doi: 10.1097/TP.0000000000003538. Transplantation. 2021. PMID: 33760787 No abstract available.
References
-
- Tullius SG, Rabb H. Improving the supply and quality of deceased-donor organs for transplantation. N. Engl. J. Med. 2018;378:1920–1929. - PubMed
-
- Klassen DK, et al. The OPTN deceased donor potential study: implications for policy and practice. Am. J. Transpl. 2016;16:1707–1714. - PubMed
-
- Tullius SG, Milford E. Kidney allocation and the aging immune response. N. Engl. J. Med. 2011;364:1369–1370. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

