Brain-gut-microbiome interactions in obesity and food addiction
- PMID: 32855515
- PMCID: PMC7841622
- DOI: 10.1038/s41575-020-0341-5
Brain-gut-microbiome interactions in obesity and food addiction
Abstract
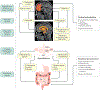
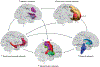
Normal eating behaviour is coordinated by the tightly regulated balance between intestinal and extra-intestinal homeostatic and hedonic mechanisms. By contrast, food addiction is a complex, maladaptive eating behaviour that reflects alterations in brain-gut-microbiome (BGM) interactions and a shift of this balance towards hedonic mechanisms. Each component of the BGM axis has been implicated in the development of food addiction, with both brain to gut and gut to brain signalling playing a role. Early-life influences can prime the infant gut microbiome and brain for food addiction, which might be further reinforced by increased antibiotic usage and dietary patterns throughout adulthood. The ubiquitous availability and marketing of inexpensive, highly palatable and calorie-dense food can further shift this balance towards hedonic eating through both central (disruptions in dopaminergic signalling) and intestinal (vagal afferent function, metabolic endotoxaemia, systemic immune activation, changes to gut microbiome and metabolome) mechanisms. In this Review, we propose a systems biology model of BGM interactions, which incorporates published reports on food addiction, and provides novel insights into treatment targets aimed at each level of the BGM axis.
Figures
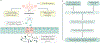


References
-
- CDC, C. f. D. C. a. P. Overweight and Obesity, <http://www.cdc.gov/obesity/data/adult.html> (2014).
-
- http://www.who.int/mediacentre/factsheets/fs311/en/. Obesity and overweight. Fact sheet, 2016).
-
- http://stateofobesity.org/rates/. Obesity Rates & Trends, 2016).

