Acyclic Cucurbit[n]uril-Type Containers as Receptors for Neuromuscular Blocking Agents: Structure-Binding Affinity Relationships
- PMID: 32855560
- PMCID: PMC7449143
- DOI: 10.5562/cca3507
Acyclic Cucurbit[n]uril-Type Containers as Receptors for Neuromuscular Blocking Agents: Structure-Binding Affinity Relationships
Abstract
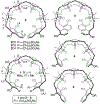
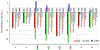
Acyclic cucurbit[n]uril molecular containers 1 and 2C3 have previously been shown to strongly bind to the neuromuscular blocking agents rocuronium, vecuronium, pancuronium, and cisatracurium in vitro by optical methods and to reverse neuromuscular block in vivo in rats. In this paper we study the in vitro binding of a panel of acyclic CB[n]-type receptors toward the four neuromuscular blocking agents and acetylcholine to develop structure-binding affinity relationships. The selected variants include those with different aromatic sidewalls (e.g. 1Me4 with dimethyl o-xylylene walls; 3 with 1,8-linked naphthalene walls), with different glycoluril oligomer lengths (e.g. 4 and 5 based on glycoluril trimer), and with different linker lengths between aromatic wall and SO3 - solubilizing group (e.g. 2C2 - 2C4). Based on the analysis of complexation induced changes in 1H NMR chemical shift we conclude that the hydrophobic regions of the guests bind in the hydrophobic cavity of the hosts with the cationic moieties of the guest binding at the ureidyl C=O portals by ion-dipole and ion-ion interactions. The thermodynamic parameters of binding were determined by direct and competition isothermal titration calorimetry experiments. We find that hosts 4 and 5 based on glycoluril trimer form significantly weaker complexes with the streroidal NMBAs than with the analogues hosts based on glycoluril tetramer (1 and 2C3). Similarly, hosts 1Me4 and 3 with different length and height aromatic walls do not exhibit the extreme binding constants displayed by 2C3 but rather behave similarly to 1. Finally, we find that hosts 2C2 and 2C4 bind only slightly more weakly to the NMBAs than 2C3, but retain the ability to discriminate against acetylcholine, and possess higher inherent water solubility than 2C3. Host 2C4, in particular, holds potential for future in vivo applications.
Keywords: Cucurbituril; drugs; molecular container; neuromuscular blocking agent; reversal agent.
Conflict of interest statement
Disclosure statement. The University of Maryland holds patents on the use of acyclic CB[n]-type receptors in biomedical applications where L.I. is named as an inventor.
Figures
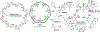

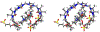

Similar articles
-
Acyclic cucurbit[n]uril congeners are high affinity hosts.J Org Chem. 2010 Jul 16;75(14):4786-95. doi: 10.1021/jo100760g. J Org Chem. 2010. PMID: 20540586
-
Acyclic Cucurbit[n]uril-type Receptors: Preparation, Molecular Recognition Properties and Biological Applications.Isr J Chem. 2018 Apr;58(3-4):250-263. doi: 10.1002/ijch.201700098. Epub 2017 Nov 14. Isr J Chem. 2018. PMID: 29805180 Free PMC article.
-
Acyclic cucurbit[n]uril-type molecular containers: influence of glycoluril oligomer length on their function as solubilizing agents.Org Biomol Chem. 2015 Apr 7;13(13):4041-50. doi: 10.1039/c5ob00184f. Org Biomol Chem. 2015. PMID: 25731639 Free PMC article.
-
Cucurbit[n]uril type hosts for the reversal of steroidal neuromuscular blocking agents.Future Med Chem. 2013 Nov;5(17):2075-89. doi: 10.4155/fmc.13.164. Future Med Chem. 2013. PMID: 24215347 Review.
-
The cucurbit[n]uril family.Angew Chem Int Ed Engl. 2005 Aug 5;44(31):4844-70. doi: 10.1002/anie.200460675. Angew Chem Int Ed Engl. 2005. PMID: 16052668 Review.
Cited by
-
A novel method for drug-target interaction prediction based on graph transformers model.BMC Bioinformatics. 2022 Nov 3;23(1):459. doi: 10.1186/s12859-022-04812-w. BMC Bioinformatics. 2022. PMID: 36329406 Free PMC article.
-
Supramolecular nanotherapeutics based on cucurbiturils.J Nanobiotechnology. 2024 Dec 23;22(1):790. doi: 10.1186/s12951-024-03024-z. J Nanobiotechnology. 2024. PMID: 39710716 Free PMC article. Review.
-
Acyclic Cucurbit[n]uril-Type Receptors: Aromatic Wall Extension Enhances Binding Affinity, Delivers Helical Chirality, and Enables Fluorescence Sensing.Chemistry. 2020 Nov 26;26(66):15249-15258. doi: 10.1002/chem.202002874. Epub 2020 Oct 16. Chemistry. 2020. PMID: 32658342 Free PMC article.
-
When Molecules Meet in Water-Recent Contributions of Supramolecular Chemistry to the Understanding of Molecular Recognition Processes in Water.ChemistryOpen. 2022 Apr;11(4):e202200028. doi: 10.1002/open.202200028. ChemistryOpen. 2022. PMID: 35373466 Free PMC article. Review.
-
Overcoming barriers with non-covalent interactions: supramolecular recognition of adamantyl cucurbit[n]uril assemblies for medical applications.RSC Med Chem. 2023 Dec 6;15(2):433-471. doi: 10.1039/d3md00596h. eCollection 2024 Feb 21. RSC Med Chem. 2023. PMID: 38389878 Free PMC article. Review.
References
-
- ) Bom A; Bradley M; Cameron K; Clark JK; Van Egmond J; Feilden H; MacLean EJ; Muir AW; Palin R; Rees DC; Zhang M-Q, Angew. Chem., Int. Ed. 2002, 41, 265. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
