Rhodiola Crenulata ameliorates exhaustive exercise-induced fatigue in mice by suppressing mitophagy in skeletal muscle
- PMID: 32855685
- PMCID: PMC7444336
- DOI: 10.3892/etm.2020.9072
Rhodiola Crenulata ameliorates exhaustive exercise-induced fatigue in mice by suppressing mitophagy in skeletal muscle
Abstract
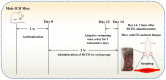
The aim of present study was to evaluate the potential effects of Rhodiola crenulata oral liquid (RCOL) on exhaustive exercise (EE)-induced fatigue in mice. Male Institute of Cancer Research mice from five treatment groups (n=10 per group) were orally administered with sterilized water for the Control and EE groups and/or RCOL at doses of 1.02, 3.03 and 6.06 ml/kg/day, once daily for 2 weeks. Anti-fatigue activity was subsequently evaluated by measuring the levels of creatine kinase (CK), lactic acid (LA), lactate dehydrogenase (LDH), malondialdehyde (MDA), superoxide dismutase (SOD), catalase (CAT) and total anti-oxidative capability (T-AOC). Histopathology was assessed using hematoxylin and eosin staining. Ultrastructures of mitochondria were observed by transmission electron microscopy. Energy supply capacity was assessed using citrate synthase (CS), succinate dehydrogenase (SDH), Na+-K+-ATPase, and liver and quadriceps glycogen content assays. Expression levels of mRNA and protein associated with mitophagy in the skeletal muscle were measured by reverse transcription-quantitative PCR and western blotting, respectively. RCOL was observed to markedly inhibit fatigue-induced oxidative stress by increasing the activities of SOD, CAT and T-AOC, whilst reducing the accumulation of LA, CK, LDH and MDA. Histological analysis of the quadriceps femoris tissue suggested increased numbers of muscle fibers in the RCOL groups compared with those in the EE group. RCOL administration was found to reverse EE-induced mitochondrial structural damage and alleviated defects inflicted onto the energy supply mechanism by increasing CS, SDH, Na+-K+-ATPase and glycogen levels. Additionally, RCOL reduced the protein expression of PTEN-induced kinase 1 (PINK1), Parkin, microtubule-associated proteins 1A/1B light chain 3, sequestosome 1 and ubiquitin, whilst lowering the gene expression of PINK1 and Parkin. Taken together, results from the present study clarified the anti-fatigue effect of RCOL, where the underlying mechanism may be associated with increased antioxidant activity, enhanced energy production and the inhibition of mitophagy by suppressing the PINK1/Parkin signaling pathway.
Keywords: Rhodiola crenulata oral liquid; anti-fatigue; loaded swimming; mitophagy; quadriceps femoris.
Copyright: © Hou et al.
Figures




References
-
- Kaufman JL, Mina R, Jakubowiak AJ, Zimmerman TL, Wolf JJ, Lewis C, Gleason C, Sharp C, Martin T, Heffner LT, et al. Combining carfilzomib and panobinostat to treat relapsed/refractory multiple myeloma: Results of a multiple myeloma research consortium Phase I Study. Blood Cancer J. 2019;9(3) doi: 10.1038/s41408-018-0154-8. - DOI - PMC - PubMed
-
- Witlox L, Hiensch AE, Velthuis MJ, Steins Bisschop CN, Los M, Erdkamp FL, Bloemendal HJ, Verhaar M, Ten Bokkel Huinink D, van der Wall E, et al. Four-year effects of exercise on fatigue and physical activity in patients with cancer. BMC Med. 2018;16(86) doi: 10.1186/s12916-018-1075-x. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
