Evolution of lifestyles in Capnodiales
- PMID: 32855743
- PMCID: PMC7426231
- DOI: 10.1016/j.simyco.2020.02.004
Evolution of lifestyles in Capnodiales
Abstract
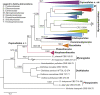
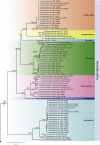
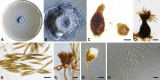
The Capnodiales, which includes fungi known as the sooty moulds, represents the second largest order in Dothideomycetes, encompassing morphologically and ecologically diverse fungi with different lifestyles and modes of nutrition. They include saprobes, plant and human pathogens, mycoparasites, rock-inhabiting fungi (RIF), lichenised, epi-, ecto- and endophytes. The aim of this study was to elucidate the lifestyles and evolutionary patterns of the Capnodiales as well as to reconsider their phylogeny by including numerous new collections of sooty moulds, and using four nuclear loci, LSU, ITS, TEF-1α and RPB2. Based on the phylogenetic results, combined with morphology and ecology, Capnodiales s. lat. is shown to be polyphyletic, representing seven different orders. The sooty moulds are restricted to Capnodiales s. str., while Mycosphaerellales is resurrected, and five new orders including Cladosporiales, Comminutisporales, Neophaeothecales, Phaeothecales and Racodiales are introduced. Four families, three genera, 21 species and five combinations are introduced as new. Furthermore, ancestral reconstruction analysis revealed that the saprobic lifestyle is a primitive state in Capnodiales s. lat., and that several transitions have occurred to evolve lichenised, plant and human parasitic, ectophytic (sooty blotch and flyspeck) and more recently epiphytic (sooty mould) lifestyles.
Keywords: Capnodiales; Capnodium alfenasii Abdollahz. & Crous; Capnodium blackwelliae Abdollahz. & Crous; Capnodium gamsii Abdollahz. & Crous; Capnodium neocoffeicola Abdollahz. & Crous; Capnodium paracoffeicola Abdollahz. & Crous; Chaetocapnodium indonesiacum Abdollahz. & Crous; Chaetocapnodium insulare Abdollahz. & Crous; Chaetocapnodium philippinense (Hongsanan & K.D. Hyde) Abdollahz. & Crous; Chaetocapnodium placitae (Cheewangkoon & Crous) Abdollahz. & Crous; Chaetocapnodium summerellii Abdollahz. & Crous; Chaetocapnodium tanzanicum Abdollahz. & Crous; Chaetocapnodium thailandense Abdollahz. & Crous; Cladosporiales Abdollahz. & Crous; Cladosporium; Comminutisporaceae Abdollahz. & Crous; Comminutisporales Abdollahz. & Crous; Leptoxyphium citri Abdollahz. & Crous; Multigene phylogeny; Mycosphaerella; Neoantennariella Abdollahz. & Crous; Neoantennariella phylicae Abdollahz. & Crous; Neoantennariellaceae Abdollahz. & Crous; Neoasbolisia Abdollahz. & Crous; Neoasbolisia phylicae Abdollahz. & Crous; Neophaeotheca Abdollahz. & Crous; Neophaeotheca salicorniae (Crous & Roets) Abdollahz. & Crous; Neophaeotheca triangularis (de Hoog & Beguin) Abdollahz. & Crous; Neophaeothecaceae Abdollahz. & Crous; Neophaeothecales Abdollahz. & Crous; Phaeothecales Abdollahz. & Crous; Phaeoxyphiella australiana Abdollahz. & Crous; Phaeoxyphiella phylicae Abdollahz. & Crous; Phragmocapnias plumeriae (Hongsanan & K.D. Hyde) Abdollahz. & Crous; Racodiales Abdollahz. & Crous; Readerielliopsidaceae Abdollahz. & Crous; Scolecoxyphium blechni Abdollahz. & Crous; Scolecoxyphium blechnicola Abdollahz. & Crous; Scolecoxyphium leucadendri Abdollahz. & Crous; Scolecoxyphium phylicae Abdollahz. & Crous; Scorias aphidis Abdollahz. & Crous; Scorias camelliae Abdollahz. & Crous; Sooty moulds.
© 2020 Westerdijk Fungal Biodiversity Institute. Production and hosting by ELSEVIER B.V.
Figures






















References
-
- Aptroot A. CBS-KNAW Fungal Biodiversity Centre; Utrecht, The Netherlands: 2006. Mycosphaerella and its anamorphs 2. Conspectus of mycosphaerella; pp. 1–231. CBS Biodiversity Series 5.
-
- Batista A.C., Ciferri R. Capnodiales. Saccardoa. 1963;2:1–296.
-
- Batista A.C., Ciferri R. The sooty-molds of the family Asbolisiaceae. Quaderno del Laboratorio Crittogamico del Istituto Botanico dell'Università di Pavia. 1963;31:1–229.
LinkOut - more resources
Full Text Sources
Research Materials
