A Review of Deep Learning for Screening, Diagnosis, and Detection of Glaucoma Progression
- PMID: 32855846
- PMCID: PMC7424906
- DOI: 10.1167/tvst.9.2.42
A Review of Deep Learning for Screening, Diagnosis, and Detection of Glaucoma Progression
Abstract
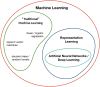
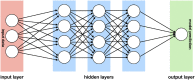
Because of recent advances in computing technology and the availability of large datasets, deep learning has risen to the forefront of artificial intelligence, with performances that often equal, or sometimes even exceed, those of human subjects on a variety of tasks, especially those related to image classification and pattern recognition. As one of the medical fields that is highly dependent on ancillary imaging tests, ophthalmology has been in a prime position to witness the application of deep learning algorithms that can help analyze the vast amount of data coming from those tests. In particular, glaucoma stands as one of the conditions where application of deep learning algorithms could potentially lead to better use of the vast amount of information coming from structural and functional tests evaluating the optic nerve and macula. The purpose of this article is to critically review recent applications of deep learning models in glaucoma, discussing their advantages but also focusing on the challenges inherent to the development of such models for screening, diagnosis and detection of progression. After a brief general overview of deep learning and how it compares to traditional machine learning classifiers, we discuss issues related to the training and validation of deep learning models and how they specifically apply to glaucoma. We then discuss specific scenarios where deep learning has been proposed for use in glaucoma, such as screening with fundus photography, and diagnosis and detection of glaucoma progression with optical coherence tomography and standard automated perimetry.
Translational relevance: Deep learning algorithms have the potential to significantly improve diagnostic capabilities in glaucoma, but their application in clinical practice requires careful validation, with consideration of the target population, the reference standards used to build the models, and potential sources of bias.
Keywords: deep learning; glaucoma; optical coherence tomography; visual fields.
Copyright 2020 The Authors.
Conflict of interest statement
Disclosure: A.C. Thompson, None; A.A. Jammal, None; F.A. Medeiros, Aeri Pharmaceuticals (C); Allergan (C, F), Annexon (C); Biogen (C); Carl Zeiss Meditec (C, F), Galimedix (C); Google Inc. (F); Heidelberg Engineering (F), IDx (C); nGoggle Inc. (P), Novartis (F); Stealth Biotherapeutics (C); Reichert (C, F)
Figures


References
-
- Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014; 121: 2081–2090. - PubMed
-
- Hennis A, Wu SY, Nemesure B, Honkanen R, Leske MC, Barbados Eye Studies G. Awareness of incident open-angle glaucoma in a population study: the Barbados Eye Studies. Ophthalmology. 2007; 114: 1816–1821. - PubMed
-
- Harwerth RS, Carter-Dawson L, Smith Barnes ELG III, Holt WF, Crawford MLJ. Neural Losses Correlated with Visual Losses in Clinical Perimetry. Invest Ophthalmol Vis Sci. 2004; 45: 3152–3160. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

