A Flow Cytometric Method to Determine Transfection Efficiency
- PMID: 32855997
- PMCID: PMC7449265
- DOI: 10.21769/bioprotoc.3244
A Flow Cytometric Method to Determine Transfection Efficiency
Abstract
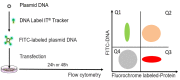
Mammalian cell transfection is a powerful technique commonly used in molecular biology to express exogenous DNA or RNA in cells and study gene and protein function. Although several transfection strategies have been developed, there is a wide variation with regards to transfection efficiency, cell toxicity and reproducibility. Thus, a sensitive and robust method that can optimize transfection efficiency based not only on expression of the target protein of interest but also on the uptake of the nucleic acids, can be an important tool in molecular biology. Herein, we present a simple, rapid and robust flow cytometric method that can be used as a tool to optimize transfection efficiency while overcoming limitations of prior established methods that quantify transfection efficiency.
Keywords: DNA labeling; Flow cytometry; Nucleic acids; Protein expression; Transfection.
Conflict of interest statement
Competing Interests The authors have declared that no competing interests exist.
Figures

References
-
- Stoll S. M. and Calos M. P.(2002). Extrachromosomal plasmid vectors for gene therapy. Curr Opin Mol Ther 4(4): 299-305. - PubMed
-
- Homann S., Hofmann C., Gorin A. M., Nguyen H. C. X., Huynh D., Hamid P., Maithel N., Yacoubian V., Mu W., Kossyvakis A., Sen Roy S., Yang O. O. and Kelesidis T.(2017). A novel rapid and reproducible flow cytometric method for optimization of transfection efficiency in cells. PLoS One 12(9): e0182941. - PMC - PubMed

