Concepts in immuno-oncology: tackling B cell malignancies with CD19-directed bispecific T cell engager therapies
- PMID: 32856140
- PMCID: PMC7481145
- DOI: 10.1007/s00277-020-04221-0
Concepts in immuno-oncology: tackling B cell malignancies with CD19-directed bispecific T cell engager therapies
Abstract
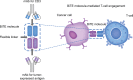
The B cell surface antigen CD19 is a target for treating B cell malignancies, such as B cell precursor acute lymphoblastic leukemia and B cell non-Hodgkin lymphoma. The BiTE® immuno-oncology platform includes blinatumomab, which is approved for relapsed/refractory B cell precursor acute lymphoblastic leukemia and B cell precursor acute lymphoblastic leukemia with minimal residual disease. Blinatumomab is also being evaluated in combination with other agents (tyrosine kinase inhibitors, checkpoint inhibitors, and chemotherapy) in various treatment settings, including frontline protocols. An extended half-life BiTE molecule is also under investigation. Patients receiving blinatumomab may experience cytokine release syndrome and neurotoxicity; however, these events may be less frequent and severe than in patients receiving other CD19-targeted immunotherapies, such as chimeric antigen receptor T cell therapy. We review BiTE technology for treating malignancies that express CD19, analyzing the benefits and limitations of this bispecific T cell engager platform from clinical experience with blinatumomab.
Keywords: Acute lymphoblastic leukemia; B cell malignancies; BiTE molecule; Bispecific T cell engager; Blinatumomab; CD19; Non-Hodgkin lymphoma.
Conflict of interest statement
AV receives consulting fees from Amgen, Roche, Kite/Gilead, and Novartis and has worked with Janssen. FL received honoraria from Amgen for participating in advisory boards and for a speakers’ bureau. EJ receives research grants and consults for Amgen, AbbVie, Adaptive Biotechnologies, Bristol-Myers Squibb, Pfizer, and Takeda. JS and FZ are employees and stockholders of Amgen Inc.
Figures
Similar articles
-
Efficacy and safety of bispecific T-cell engager blinatumomab and the potential to improve leukemia-free survival in B-cell acute lymphoblastic leukemia.Expert Rev Hematol. 2017 Dec;10(12):1057-1067. doi: 10.1080/17474086.2017.1396890. Epub 2017 Nov 1. Expert Rev Hematol. 2017. PMID: 29082835 Review.
-
Treatment with anti CD19 chimeric antigen receptor T cells after antibody-based immunotherapy in adults with acute lymphoblastic leukemia.Curr Res Transl Med. 2020 Jan;68(1):17-22. doi: 10.1016/j.retram.2019.12.001. Epub 2019 Dec 25. Curr Res Transl Med. 2020. PMID: 31882377 Review.
-
CAR T-cell therapy is effective for CD19-dim B-lymphoblastic leukemia but is impacted by prior blinatumomab therapy.Blood Adv. 2019 Nov 26;3(22):3539-3549. doi: 10.1182/bloodadvances.2019000692. Blood Adv. 2019. PMID: 31738832 Free PMC article.
-
Bispecific antibodies in acute lymphoblastic leukemia therapy.Expert Rev Hematol. 2020 Nov;13(11):1211-1233. doi: 10.1080/17474086.2020.1831380. Epub 2020 Nov 4. Expert Rev Hematol. 2020. PMID: 33000968
-
The BiTE (bispecific T-cell engager) platform: Development and future potential of a targeted immuno-oncology therapy across tumor types.Cancer. 2020 Jul 15;126(14):3192-3201. doi: 10.1002/cncr.32909. Epub 2020 May 13. Cancer. 2020. PMID: 32401342 Review.
Cited by
-
Advances in new targets for immunotherapy of small cell lung cancer.Thorac Cancer. 2024 Jan;15(1):3-14. doi: 10.1111/1759-7714.15178. Epub 2023 Dec 13. Thorac Cancer. 2024. PMID: 38093497 Free PMC article. Review.
-
Immunotargets and Therapy for Systemic Lupus Erythematosus.Immunotargets Ther. 2025 Jun 24;14:605-629. doi: 10.2147/ITT.S485650. eCollection 2025. Immunotargets Ther. 2025. PMID: 40585390 Free PMC article. Review.
-
Current and emerging monoclonal antibodies, antibody-drug conjugates, and bispecific antibodies in treatment of lymphoma.Leuk Res Rep. 2022 Apr 28;17:100319. doi: 10.1016/j.lrr.2022.100319. eCollection 2022. Leuk Res Rep. 2022. PMID: 35539019 Free PMC article.
-
Blinatumomab as a Bridge Therapy for Hematopoietic Stem Cell Transplantation in Pediatric Refractory/Relapsed Acute Lymphoblastic Leukemia.Cancers (Basel). 2022 Jan 17;14(2):458. doi: 10.3390/cancers14020458. Cancers (Basel). 2022. PMID: 35053619 Free PMC article.
-
Advances in DLL3-targeted therapies for small cell lung cancer: challenges, opportunities, and future directions.Front Oncol. 2024 Dec 5;14:1504139. doi: 10.3389/fonc.2024.1504139. eCollection 2024. Front Oncol. 2024. PMID: 39703856 Free PMC article. Review.
References
-
- Morphosys (2020) Tafasitamab (MOR208). https://www.morphosys.com/pipeline/proprietary-portfolio/tafasitamab-mor208. Accessed 9 March 2020
-
- American Cancer Society Typical treatment of acute lymphocytic leukemia (ALL). https://www.cancer.org/cancer/acute-lymphocytic-leukemia/treating/typica.... Accessed July 11 2019
-
- American Cancer Society Targeted therapy for acute lymphocytic leukemia (ALL). https://www.cancer.org/cancer/acute-lymphocytic-leukemia/treating/target.... Accessed July 11 2019
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources