Two-Photon Polymerisation 3D Printing of Microneedle Array Templates with Versatile Designs: Application in the Development of Polymeric Drug Delivery Systems
- PMID: 32856172
- PMCID: PMC7452932
- DOI: 10.1007/s11095-020-02887-9
Two-Photon Polymerisation 3D Printing of Microneedle Array Templates with Versatile Designs: Application in the Development of Polymeric Drug Delivery Systems
Abstract
Purpose: To apply a simple and flexible manufacturing technique, two-photon polymerisation (2PP), to the fabrication of microneedle (MN) array templates with high precision and low cost in a short time.
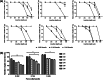
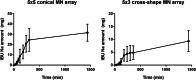
Methods: Seven different MN array templates were produced by 2PP 3D printing, varying needle height (900-1300 μm), shape (conical, pyramidal, cross-shaped and with pedestal), base width (300-500 μm) and interspacing (100-500 μm). Silicone MN array moulds were fabricated from these templates and used to produce dissolving and hydrogel-forming MN arrays. These polymeric MN arrays were evaluated for their insertion in skin models and their ability to deliver model drugs (cabotegravir sodium and ibuprofen sodium) to viable layers of the skin (ex vivo and in vitro) for subsequent controlled release and/or absorption.
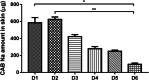
Results: The various templates obtained with 2PP 3D printing allowed the reproducible fabrication of multiple MN array moulds. The polymeric MN arrays produced were efficiently inserted into two different skin models, with sharp conical and pyramidal needles showing the highest insertion depth values (64-90% of needle height). These results correlated generally with ex vivo and in vitro drug delivery results, where the same designs showed higher drug delivery rates after 24 h of application.
Conclusion: This work highlights the benefits of using 2PP 3D printing to prototype variable MN array designs in a simple and reproducible manner, for their application in drug delivery.
Keywords: 3D printing; dissolving; hydrogel-forming; microneedle array; two-photon polymerisation.
Figures






Similar articles
-
Optimization of stereolithography 3D printing of microneedle micro-molds for ocular drug delivery.Int J Pharm. 2024 Jun 10;658:124195. doi: 10.1016/j.ijpharm.2024.124195. Epub 2024 May 3. Int J Pharm. 2024. PMID: 38703935
-
Fabrication and mechanical/biological evaluations of dissolving bird-bill microneedle arrays.Drug Deliv Transl Res. 2025 Jul;15(7):2581-2588. doi: 10.1007/s13346-024-01757-w. Epub 2024 Dec 9. Drug Deliv Transl Res. 2025. PMID: 39653959 Free PMC article.
-
Influence of array interspacing on the force required for successful microneedle skin penetration: theoretical and practical approaches.J Pharm Sci. 2013 Apr;102(4):1209-21. doi: 10.1002/jps.23439. Epub 2013 Jan 28. J Pharm Sci. 2013. PMID: 23359221
-
3D printing applications for transdermal drug delivery.Int J Pharm. 2018 Jun 15;544(2):415-424. doi: 10.1016/j.ijpharm.2018.01.031. Epub 2018 Jan 20. Int J Pharm. 2018. PMID: 29355656 Review.
-
A key role by polymers in microneedle technology: a new era.Drug Dev Ind Pharm. 2021 Nov;47(11):1713-1732. doi: 10.1080/03639045.2022.2058531. Epub 2022 Apr 6. Drug Dev Ind Pharm. 2021. PMID: 35332822 Review.
Cited by
-
A Review of 3D-Printing of Microneedles.Pharmaceutics. 2022 Dec 1;14(12):2693. doi: 10.3390/pharmaceutics14122693. Pharmaceutics. 2022. PMID: 36559187 Free PMC article. Review.
-
3D-Printed Products for Topical Skin Applications: From Personalized Dressings to Drug Delivery.Pharmaceutics. 2021 Nov 17;13(11):1946. doi: 10.3390/pharmaceutics13111946. Pharmaceutics. 2021. PMID: 34834360 Free PMC article. Review.
-
Enhancing melanoma therapy with hydrogel microneedles.Front Oncol. 2025 Apr 17;15:1590534. doi: 10.3389/fonc.2025.1590534. eCollection 2025. Front Oncol. 2025. PMID: 40313257 Free PMC article. Review.
-
Advances in the development of microarray patches in biomedicine.N Biotechnol. 2025 May 25;86:25-30. doi: 10.1016/j.nbt.2025.01.003. Epub 2025 Jan 14. N Biotechnol. 2025. PMID: 39814261 Review.
-
Quality-by-design principles applied to the development and optimisation of lidocaine-loaded dissolving microneedle arrays - a proof-of-concept.Drug Deliv Transl Res. 2025 Aug;15(8):2643-2662. doi: 10.1007/s13346-024-01758-9. Epub 2025 Jan 3. Drug Deliv Transl Res. 2025. PMID: 39751764
References
-
- Prausnitz MR. Engineering microneedle patches for vaccination and drug delivery to skin. Annu Rev Chem Biomol Eng. 2017;8(1):9.1–9.24. - PubMed
-
- Larrañeta E, Lutton RE, Woolfson AD, Donnelly RF. Microneedle arrays as transdermal and intradermal drug delivery systems: materials science, manufacture and commercial development. Mater Sci Eng R Rep. 2016;104:1–32.
-
- Eltayib E, Brady AJ, Caffarel-Salvador E, González-Vázquez P, Zaid Alkilani A, McCarthy HO, et al. Hydrogel-forming microneedle arrays: potential for use in minimally-invasive lithium monitoring. Eur J Pharm Biopharm. 2016;102:123–131. - PubMed
-
- Donnelly RF, Mooney K, Caffarel-Salvador E, Torrisi BM, Eltayib E, McElnay JC. Microneedle-mediated minimally invasive patient monitoring. Ther Drug Monit. 2013;36(1):10–17. - PubMed
-
- Babity S, Roohnikan M, Brambilla D. Advances in the design of transdermal microneedles for diagnostic and monitoring applications. Small. 2018;14(49):1–16. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

