Multinational Association of Supportive Care in Cancer (MASCC) 2020 clinical practice recommendations for the management of severe dermatological toxicities from checkpoint inhibitors
- PMID: 32856211
- PMCID: PMC8996262
- DOI: 10.1007/s00520-020-05706-4
Multinational Association of Supportive Care in Cancer (MASCC) 2020 clinical practice recommendations for the management of severe dermatological toxicities from checkpoint inhibitors
Abstract
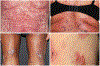
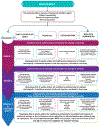
Immune checkpoint inhibitors (ICIs) frequently result in cutaneous immune-related adverse events (IrAEs). Although the majority of these events are mild-to-moderate in severity, up to 5% are severe, which may lead to morbidity and dose interruption or discontinuation of ICI therapy. In addition, up to 25% of dermatologic IrAEs are corticosteroid-refractory or corticosteroid-dependent. These 2020 MASCC recommendations cover the diagnosis and management of cutaneous IrAEs with a focus on moderate-to-severe and corticosteroid-resistant events. Although the usage of immune-suppressive therapy has been advocated in this setting, there is a lack of randomized clinical trial data to provide a compelling level of evidence of its therapeutic benefit.
Keywords: Bullous dermatoses; Corticosteroids; Cutaneous IrAEs; Inflammatory dermatitis; Pruritus; Skin rash; Vitiligo.
Figures
References
-
- Boutros C, Tarhini A, Routier E, Lambotte O, Ladurie FL, Carbonnel F, Izzeddine H, Marabelle A, Champiat S, Berdelou A, Lanoy E, Texier M, Libenciuc C, Eggermont AM, Soria JC, Mateus C, Robert C (2016) Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol 13(8):473–486. doi: 10.1038/nrclinonc.2016.58. - DOI - PubMed
-
- Puzanov I, Diab A, Abdallah K, Bingham CO 3rd, Brogdon C, Dadu R, Hamad L, Kim S, Lacouture ME, LeBoeuf NR, Lenihan D, Onofrei C, Shannon V, Sharma R, Silk AW, Skondra D, Suarez-Almazor ME, Wang Y, Wiley K, Kaufman HL, Ernstoff MS (2017) Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer 5(1):95. doi: 10.1186/s40425-017-0300-z. - DOI - PMC - PubMed
-
- Hassel JC, Heinzerling L, Aberle J, Bähr O, Eigentler TK, Grimm MO, Grünwald V, Leipe J, Reinmuth N, Tietze JK, Trojan J, Zimmer L, Gutzmer R (2017) Combined immune checkpoint blockade (anti-PD-1/anti-CTLA-4): Evaluation and management of adverse drug reactions. Cancer Treat Rev 57:36–49. doi: 10.1016/j.ctrv.2017.05.003. - DOI - PubMed
-
- Haanen J, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, Jordan K; ESMO Guidelines Committee (2017) Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 28(suppl_4):iv119–iv142. doi: 10.1093/annonc/mdx225. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

