Roles of MicroRNA-122 in Cardiovascular Fibrosis and Related Diseases
- PMID: 32856216
- PMCID: PMC7451782
- DOI: 10.1007/s12012-020-09603-4
Roles of MicroRNA-122 in Cardiovascular Fibrosis and Related Diseases
Abstract
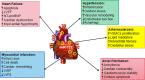
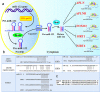
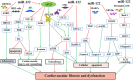
Fibrotic diseases cause annually more than 800,000 deaths worldwide, where of the majority accounts for cardiovascular fibrosis, which is characterized by endothelial dysfunction, myocardial stiffening and reduced dispensability. MicroRNAs (miRs), small noncoding RNAs, play critical roles in cardiovascular dysfunction and related disorders. Intriguingly, there is a critical link among miR-122, cardiovascular fibrosis, sirtuin 6 (SIRT6) and angiotensin-converting enzyme 2 (ACE2), which was recently identified as a coreceptor for SARS-CoV2 and a negative regulator of the rennin-angiotensin system. MiR-122 overexpression appears to exacerbate the angiotensin II-mediated loss of autophagy and increased inflammation, apoptosis, extracellular matrix deposition, cardiovascular fibrosis and dysfunction by modulating the SIRT6-Elabela-ACE2, LGR4-β-catenin, TGFβ-CTGF and PTEN-PI3K-Akt signaling pathways. More importantly, the inhibition of miR-122 has proautophagic, antioxidant, anti-inflammatory, anti-apoptotic and antifibrotic effects. Clinical and experimental studies clearly demonstrate that miR-122 functions as a crucial hallmark of fibrogenesis, cardiovascular injury and dysfunction. Additionally, the miR-122 level is related to the severity of hypertension, atherosclerosis, atrial fibrillation, acute myocardial infarction and heart failure, and miR-122 expression is a risk factor for these diseases. The miR-122 level has emerged as an early-warning biomarker cardiovascular fibrosis, and targeting miR-122 is a novel therapeutic approach against progression of cardiovascular dysfunction. Therefore, an increased understanding of the cardiovascular roles of miR-122 will help the development of effective interventions. This review summarizes the biogenesis of miR-122; regulatory effects and underlying mechanisms of miR-122 on cardiovascular fibrosis and related diseases; and its function as a potential specific biomarker for cardiovascular dysfunction.
Keywords: ACE2; Cardiovascular dysfunction; Fibrosis; Sirtuin 6; microRNA-122.
Conflict of interest statement
All the authors declare that they have no conflict of interest.
Figures
Similar articles
-
MicroRNA-122-5p Aggravates Angiotensin II-Mediated Myocardial Fibrosis and Dysfunction in Hypertensive Rats by Regulating the Elabela/Apelin-APJ and ACE2-GDF15-Porimin Signaling.J Cardiovasc Transl Res. 2022 Jun;15(3):535-547. doi: 10.1007/s12265-022-10214-3. Epub 2022 Feb 16. J Cardiovasc Transl Res. 2022. PMID: 35174434 Free PMC article.
-
MicroRNA-122 aggravates angiotensin II-mediated apoptosis and autophagy imbalance in rat aortic adventitial fibroblasts via the modulation of SIRT6-elabela-ACE2 signaling.Eur J Pharmacol. 2020 Sep 15;883:173374. doi: 10.1016/j.ejphar.2020.173374. Epub 2020 Jul 16. Eur J Pharmacol. 2020. PMID: 32682786 Free PMC article.
-
Targeting the microRNA-34a as a Novel Therapeutic Strategy for Cardiovascular Diseases.Front Cardiovasc Med. 2022 Jan 27;8:784044. doi: 10.3389/fcvm.2021.784044. eCollection 2021. Front Cardiovasc Med. 2022. PMID: 35155600 Free PMC article. Review.
-
Inhibitory effect of microRNA-21 on pathways and mechanisms involved in cardiac fibrosis development.Ther Adv Cardiovasc Dis. 2024 Jan-Dec;18:17539447241253134. doi: 10.1177/17539447241253134. Ther Adv Cardiovasc Dis. 2024. PMID: 38819836 Free PMC article. Review.
-
MicroRNA-21 in cardiovascular disease.J Cardiovasc Transl Res. 2010 Jun;3(3):251-5. doi: 10.1007/s12265-010-9169-7. Epub 2010 May 1. J Cardiovasc Transl Res. 2010. PMID: 20560046 Free PMC article. Review.
Cited by
-
Regulation of Nrf2 signaling pathway in heart failure: Role of extracellular vesicles and non-coding RNAs.Free Radic Biol Med. 2021 May 1;167:218-231. doi: 10.1016/j.freeradbiomed.2021.03.013. Epub 2021 Mar 17. Free Radic Biol Med. 2021. PMID: 33741451 Free PMC article. Review.
-
MicroRNA-122-5p Aggravates Angiotensin II-Mediated Myocardial Fibrosis and Dysfunction in Hypertensive Rats by Regulating the Elabela/Apelin-APJ and ACE2-GDF15-Porimin Signaling.J Cardiovasc Transl Res. 2022 Jun;15(3):535-547. doi: 10.1007/s12265-022-10214-3. Epub 2022 Feb 16. J Cardiovasc Transl Res. 2022. PMID: 35174434 Free PMC article.
-
LOC102549726/miR-760-3p network is involved in the progression of ISO-induced pathological cardiomyocyte hypertrophy via endoplasmic reticulum stress.J Mol Histol. 2023 Dec;54(6):675-687. doi: 10.1007/s10735-023-10166-1. Epub 2023 Oct 30. J Mol Histol. 2023. PMID: 37899367 Free PMC article.
-
Regulatory Roles and Therapeutic Potential of miR-122-5p in Hypoxic-Ischemic Brain Injury: Comprehensive Review.Cell Biochem Biophys. 2025 Feb 28. doi: 10.1007/s12013-025-01686-6. Online ahead of print. Cell Biochem Biophys. 2025. PMID: 40016565 Review.
-
The Role of Circulating MicroRNAs in Cardiovascular Diseases: A Novel Biomarker for Diagnosis and Potential Therapeutic Targets?Cureus. 2024 Jul 8;16(7):e64100. doi: 10.7759/cureus.64100. eCollection 2024 Jul. Cureus. 2024. PMID: 39114238 Free PMC article. Review.
References
-
- Song JJ, Yang M, Liu Y, Song JW, Wang J, Chi HJ, et al. MicroRNA-122 aggravates angiotensin II-mediated apoptosis and autophagy imbalance in rat aortic adventitial fibroblasts via the modulation of SIRT6-elabela-ACE2 signaling. European Journal of Pharmacology. 2020;883:173374. doi: 10.1016/j.ejphar.2020.173374. - DOI - PMC - PubMed
-
- Pinar AA, Scott TE, Huuskes BM, Tapia Cáceres FE, Kemp-Harper BK, Samuel CS. Targeting the NLRP3 inflammasome to treat cardiovascular fibrosis. Pharmacology & Therapeutics. 2020;209:107511. - PubMed
-
- Paul M, Amos Z, Wing-Kin S. Fibrosis in chronic liver disease: An update on diagnostic and treatment modalities. Drugs. 2019;79(9):903–927. - PubMed
-
- Zhao Z, Zhong L, Li P, He K, Qiu C, Zhao L, Gong J. Cholesterol impairs hepatocyte lysosomal function causing M1 polarization of macrophages via exosomal miR-122-5p. Experimental Cell Research. 2020;387(1):111738. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

