Single versus dual blockade of the renin-angiotensin system in patients with IgA nephropathy
- PMID: 32856272
- PMCID: PMC7701065
- DOI: 10.1007/s40620-020-00836-8
Single versus dual blockade of the renin-angiotensin system in patients with IgA nephropathy
Abstract
Background: Inhibitors of the renin-angiotensin system (RAS) are cornerstones of supportive therapy in patients with IgA nephropathy (IgAN). We analyzed the effects of single versus dual RAS blockaQueryde during our randomized STOP-IgAN trial.
Methods: STOP-IgAN participants with available successive information on their RAS treatment regimen and renal outcomes during the randomized 3-year trial phase were stratified post hoc into two groups, i.e. patients under continuous single or dual RAS blocker therapy over the entire 3 years of the trial phase. Primary and secondary STOP-IgAN trial endpoints, i.e. frequencies of full clinical remission, eGFR-loss ≥ 15 and ≥ 30 ml/min/1.73 m2 and ESRD onset, were analyzed by logistic regression and linear mixed effects models.
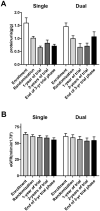
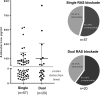
Results: Among the 112 patients included in the present analysis, 82 (73%) were maintained on single and 30 (27%) on dual RAS inhibitor therapy throughout the trial. Neither RAS blocker strategy significantly affected full clinical remission, eGFR-loss rates, onset of ESRD. Proteinuria moderately increased in patients under dual RAS blockade by 0.1 g/g creatinine during the 3-year trial phase. This was particularly evident in patients without additional immunosuppression during the randomized trial phase, where proteinuria increased by 0.2 g/g creatinine in the dual RAS blockade group. In contrast, proteinuria decreased in patients under single RAS blocker therapy by 0.3 g/g creatinine. The course of eGFR remained stable and did not differ between the RAS treatment strategies.
Conclusion: In the STOP-IgAN cohort, neither RAS blocker regimen altered renal outcomes. Patients on dual RAS blockade even exhibited higher proteinuria over the 3-year trial phase.
Keywords: IgA nephropathy; RAS blockers; RAS system; Renin-angiotensin system; STOP-IgAN.
Conflict of interest statement
J.F. has received consultant honoraria from Omeros, USA, Retrophin, USA and Calliditas, Sweden. The other authors declare no competing financial interests.
Figures
References
-
- KDIGO Clinical Practice Guideline for Glomerulonephritis Kidney International Supplements. 2012;2(2):142. doi: 10.1038/kisup.2012.12. - DOI
-
- Ma, F., et al., Treatment for IgA nephropathy with stage 3 or 4 chronic kidney disease: low-dose corticosteroids combined with oral cyclophosphamide. J Nephrol, 2020. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

