Oral 5-aminosalicylic acid for maintenance of remission in ulcerative colitis
- PMID: 32856298
- PMCID: PMC8094989
- DOI: 10.1002/14651858.CD000544.pub5
Oral 5-aminosalicylic acid for maintenance of remission in ulcerative colitis
Abstract
Background: Oral 5-aminosalicylic acid (5-ASA; also known as mesalazine or mesalamine) preparations were intended to avoid the adverse effects of sulfasalazine (SASP) while maintaining its therapeutic benefits. In an earlier version of this review, we found that 5-ASA drugs were more effective than placebo for maintenance of remission of ulcerative colitis (UC), but had a significant therapeutic inferiority relative to SASP. In this version, we have rerun the search to bring the review up to date.
Objectives: To assess the efficacy, dose-responsiveness, and safety of oral 5-ASA compared to placebo, SASP, or 5-ASA comparators for maintenance of remission in quiescent UC and to compare the efficacy and safety of once-daily dosing of oral 5-ASA with conventional (two or three times daily) dosing regimens.
Search methods: We performed a literature search for studies on 11 June 2019 using MEDLINE, Embase, and the Cochrane Library. In addition, we searched review articles and conference proceedings.
Selection criteria: We included randomized controlled trials with a minimum treatment duration of six months. We considered studies of oral 5-ASA therapy for treatment of participants with quiescent UC compared with placebo, SASP, or other 5-ASA formulations. We also included studies that compared once-daily 5-ASA treatment with conventional dosing of 5-ASA and 5-ASA dose-ranging studies.
Data collection and analysis: We used standard methodological procedures expected by Cochrane. The primary outcome was the failure to maintain clinical or endoscopic remission. Secondary outcomes were adherence, adverse events (AE), serious adverse events (SAE), withdrawals due to AEs, and withdrawals or exclusions after entry. Trials were separated into five comparison groups: 5-ASA versus placebo, 5-ASA versus SASP, once-daily dosing versus conventional dosing, 5-ASA (balsalazide, Pentasa, and olsalazine) versus comparator 5-ASA formulation (Asacol and Salofalk), and 5-ASA dose-ranging. We calculated the risk ratio (RR) and 95% confidence interval (CI) for each outcome. We analyzed data on an intention-to-treat basis, and used GRADE to assess the overall certainty of the evidence.
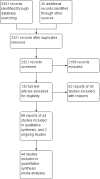
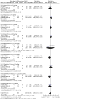
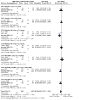
Main results: The search identified 44 studies (9967 participants). Most studies were at low risk of bias. Ten studies were at high risk of bias. Seven of these studies were single-blind and three were open-label. 5-ASA is more effective than placebo for maintenance of clinical or endoscopic remission. About 37% (335/907) of 5-ASA participants relapsed at six to 12 months compared to 55% (355/648) of placebo participants (RR 0.68, 95% CI 0.61 to 0.76; 8 studies, 1555 participants; high-certainty evidence). Adherence to study medication was not reported for this comparison. SAEs were reported in 1% (6/550) of participants in the 5-ASA group compared to 2% (5/276) of participants in the placebo group at six to 12 months (RR 0.60, 95% CI 0.19 to 1.84; 3 studies, 826 participants; low-certainty evidence). There is probably little or no difference in AEs at six to 12 months' follow-up (RR 0.93, 95% CI 0.73 to 1.18; 5 studies, 1132 participants; moderate-certainty evidence). SASP is more effective than 5-ASA for maintenance of remission. About 48% (416/871) of 5-ASA participants relapsed at six to 18 months compared to 43% (336/784) of SASP participants (RR 1.14, 95% CI 1.03 to 1.27; 12 studies, 1655 participants; high-certainty evidence). Adherence to study medication and SAEs were not reported for this comparison. There is probably little or no difference in AEs at six to 12 months' follow-up (RR 1.07, 95% CI 0.82 to 1.40; 7 studies, 1138 participants; moderate-certainty evidence). There is little or no difference in clinical or endoscopic remission rates between once-daily and conventionally dosed 5-ASA. About 37% (717/1939) of once-daily participants relapsed over 12 months compared to 39% (770/1971) of conventional-dosing participants (RR 0.94, 95% CI 0.88 to 1.01; 10 studies, 3910 participants; high-certainty evidence). There is probably little or no difference in medication adherence rates. About 10% (106/1152) of participants in the once-daily group failed to adhere to their medication regimen compared to 8% (84/1154) of participants in the conventional-dosing group (RR 1.18, 95% CI 0.72 to 1.93; 9 studies, 2306 participants; moderate-certainty evidence). About 3% (41/1587) of participants in the once-daily group experienced a SAE compared to 2% (35/1609) of participants in the conventional-dose group at six to 12 months (RR 1.20, 95% CI 0.77 to 1.87; moderate-certainty evidence). There is little or no difference in the incidence of AEs at six to 13 months' follow-up (RR 0.98, 95% CI 0.92 to 1.04; 8 studies, 3497 participants; high-certainty evidence). There may be little or no difference in the efficacy of different 5-ASA formulations. About 44% (158/358) of participants in the 5-ASA group relapsed at six to 18 months compared to 41% (142/349) of participants in the 5-ASA comparator group (RR 1.08, 95% CI 0.91 to 1.28; 6 studies, 707 participants; low-certainty evidence).
Authors' conclusions: There is high-certainty evidence that 5-ASA is superior to placebo for maintenance therapy in UC. There is high-certainty evidence that 5-ASA is inferior compared to SASP. There is probably little or no difference between 5-ASA and placebo, and 5-ASA and SASP in commonly reported AEs such as flatulence, abdominal pain, nausea, diarrhea, headache, and dyspepsia. Oral 5-ASA administered once daily has a similar benefit and harm profile as conventional dosing for maintenance of remission in quiescent UC.
Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
AM: none.
TMN: none.
CEP: none.
JKM: none.
BGF has received fees from Abbott/AbbVie, Amgen, Astra Zeneca, Avaxia Biologics Inc., Bristol‐Myers Squibb, Celgene, Centocor Inc., Elan/Biogen, Ferring, JnJ/Janssen, Merck, Nestles, Novartis, Novonordisk, Pfizer, Prometheus Laboratories, Protagonist, Salix Pharma, Takeda, Teva, TiGenix, Tillotts Pharma AG and UCB Pharma for Scientific Advisory Board membership; fees from Abbott/AbbVie, Actogenix, Akros, Albireo Pharma, Amgen, Astra Zeneca, Avaxia Biologics Inc., Avir Pharma, Axcan, Baxter Healthcare Corp., Biogen Idec, Boehringer‐Ingelheim, Bristol‐Myers Squibb, Calypso Biotech, Celgene, Elan/Biogen, EnGene, Ferring Pharma, Roche/Genentech, GiCare Pharma, Gilead, Given Imaging Inc., GSK, Ironwood Pharma, Janssen Biotech (Centocor), JnJ/Janssen, Kyowa Kakko Kirin Co Ltd., Lexicon, Lilly, Lycera BioTech, Merck, Mesoblast Pharma, Millennium, Nektar, Nestles, Novonordisk, Pfizer, Prometheus Therapeutics and Diagnostics, Protagonist, Receptos, Salix Pharma, Serono, Shire, Sigmoid Pharma, Synergy Pharma Inc., Takeda, Teva Pharma, TiGenix, Tillotts, UCB Pharma, Vertex Pharma, VHsquared Ltd., Warner‐Chilcott, Wyeth, Zealand, and Zyngenia for consultancy; payment for lectures from Abbott/AbbVie, JnJ/Janssen, Takeda, Warner‐Chilcott, UCB Pharma; his institution has received grants/grants pending from Abbott/AbbVie, Amgen, Astra Zeneca, Bristol‐Myers Squibb (BMS), Janssen Biotech (Centocor), JnJ/Janssen, Roche/Genentech, Millennium, Pfizer, Receptos, Santarus, Sanofi, Tillotts, and UCB Pharma.
Figures





























Update of
-
Oral 5-aminosalicylic acid for maintenance of remission in ulcerative colitis.Cochrane Database Syst Rev. 2016 May 9;2016(5):CD000544. doi: 10.1002/14651858.CD000544.pub4. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2020 Aug 28;8:CD000544. doi: 10.1002/14651858.CD000544.pub5. PMID: 27158764 Free PMC article. Updated.
References
References to studies included in this review
Andreoli 1987 {published data only}
-
- Andreoli A, Cosintino R, Trotti R, Berri F, Prantera C. 5-aminosalicylic acid (5-ASA) versus salazopirin (SASP) in the oral treatment of active ulcerative colitis (UC) and in remission. Clinical Controversies in Inflammatory Bowel Disease 1987:170.
Ardizzone 1995 {published data only}
-
- Ardizzone S, Petrillo M, Molteni P, Desideri S, Bianchi Porro G. Coated oral 50 aminosalicylic acid (Claversal) is equivalent to sulfasalazine for remission maintenance in ulcerative colitis. Journal of Clinical Gastroenterology 1995;21:287-9. - PubMed
-
- Bianchi Porro GB, Ardizzone S, Fasoli R, Petrillo M, Desideri S. Comparison of mesalazine with sulphasalazine in prophylactic treatment of ulcerative colitis. Gut 1989;30:A1467.
Ardizzone 1999 {published data only}
-
- Ardizzone S, Petrillo M, Imbesi V, Cerutti R, Bollani S, Bianchi Porro G. Is maintenance therapy always necessary for patients with ulcerative colitis? Alimentary Pharmacology and Therapeutics 1999;13:373-9. - PubMed
Courtney 1992 {published data only}
-
- Courtney MG, Nunes DP, Bergin CF, O'Driscoll M, Trimble V, Keeling PW, et al. Randomised comparison of olsalazine and mesalazine in prevention of relapses in ulcerative colitis. Lancet 1992;339:1279-81. - PubMed
-
- Courtney MG, Nunes DP, Bergin CF. Prospective randomized trial of mesalazine versus olsalazine in ulcerative colitis. Gastroenterology 1990;98(5 Part 2):A165.
D'Haens 2012 {published data only}
-
- D'Haens G, Sandborn WJ, Barrett K, Hodgson I, Streck P. Once-daily MMX® mesalamine for endoscopic maintenance of remission of ulcerative colitis. American Journal of Gastroenterology 2012;107(7):1064-77. - PubMed
Deventer 2001 {published data only}
-
- Deventer SJ, Hommes DW, Roskam-Mul MD, Dekker W, Gasthuis K, Wetzels A, et al. Prospective randomized open label blinded endpoint (PROBE) trial of high versus low dose mesalazine for prevention of relapse in patients with UC in remission. Gastroenterology 2001;120(5 Suppl 1):A454.
Dew 1983 {published data only}
-
- Dew MJ, Harries AD, Evans N, Evans BK, Rhodes J. Maintenance of remission in ulcerative colitis with an oral preparation of 5-aminosalicylic acid in high doses. Gut 1983;24(Suppl 10):A983.
Dignass 2009a {published data only}
-
- Bokemeyer B, Hommes D, Gill I, Broberg P, Dignass A. Mesalazine in left-sided ulcerative colitis: efficacy analyses from the PODIUM trial on maintenance of remission and mucosal healing. Journal of Crohn's and Colitis 2012;6(4):476-82. - PubMed
-
- Dignass A, Bokemeyer B, Mross M, Oudkerk Pool M. Symptom resolution and clinical remission in patients with mild-to-moderate ulcerative colitis: analysis of the PODIUM trial. Journal of Crohn's and Colitis 2013;7:S153-4.
-
- Dignass A, Bokemeyer B, Stijnen T, Klugmann T, Oudkerk M, Veerman H. Impact of once-daily versus twice-daily mesalazine (Pentasa) on relief of rectal bleeding and stool frequency: results from a multinational randomised controlled trial. In: Canadian Journal of Gastroenterology. Vol. 23. 2009.
-
- Dignass A, Bokemeyer B, Stijnen T, Tan T, Borner N, Oudkerk Pool M, et al. Safety of once-daily vs twice-daily mesalazine (Pentasa) dosing. Results from a 12-month randomised controlled trial in maintenance of remission of ulcerative colitis. Canadian Journal of Gastroenterology 2009;23:S73.
-
- Dignass A, Stijnen T, Mross MR, Vermeire S, Veerman H, Bhatt A. Pentasa (mesalazine) once or twice daily for the management of maintenance of remission of ulcerative colitis: demographic and baseline data of a 12 month single blind randomised controlled trial. Gastroenterology 2007;132(4 Suppl 1):A502-3.
Fockens 1995 {published data only}
-
- Fockens P, Mulder CJ, Ferwada J, Tytgat GN, the Dutch Pentasa Study Group. Relapse prevention of ulcerative colitis: double-blind comparison of 1.5 gram versus 3 gram oral mesalazine (Pentasa). Gastroenterology 1993;104:A701.
-
- Fockens P, Mulder CJ, Tytgat GN, Blok P, Ferwerda J, Meuwissen SG, et al. Comparison of the efficacy and safety of 1.5 compared with 3.0 g oral slow-release mesalazine (Pentasa) in the maintenance treatment of ulcerative colitis. Dutch Pentasa Study Group. European Journal of Gastroenterology and Hepatology 1995;7(11):1025-30. - PubMed
Giaffer 1992a {published data only}
-
- Giaffer MH, Holdsworth CD, Lennard-Jones JE, Rodrigues CA, McIntyre PB, Manjunatha S, et al. Improved maintenance of remission in ulcerative colitis by balsalazide 4 g/day compared with 2 g/day. Alimentary Pharmacology and Therapeutics 1992;6:479-85. - PubMed
Gordon 2016 {published data only}
-
- Gordon GL, Zakko S, Murthy U, Sedghi S, Pruitt R, Barrett AC, et al. Once-daily mesalamine formulation for maintenance of remission in ulcerative colitis. Journal of Clinical Gastroenterology 2016;50(4):318-25. - PubMed
Green 1992 {published data only}
-
- Green JR, Swan CH, Rowlinson A, Gibson JA, Brown P, Kerr GD, et al. Short report: comparison of two doses of balsalazide in maintaining ulcerative colitis in remission over 12 months. Alimentary Pharmacology and Therapeutics 1992;6:647-52. - PubMed
-
- Green JR, Swan CH, Rowlinson AE, Gibson JA, Brown P, Kerr GD, et al. A three-year prospective study of the maintenance of remission of ulcerative colitis by a new 5-ASA releasing agent, balsalazide. Gastroenterology 1992;102(4 Part 2):A631.
Green 1998 {published data only}
-
- Green JR, Gibson JA, Kerr GD, Swarbrick ET, Lobo AJ, Holdsworth CD, et al. Maintenance of remission of ulcerative colitis: a comparison between balsalzide 3 g daily and mesalazine 1.2 g daily over 12 months. Alimentary Pharmacology and Therapeutics 1998;12:1207-16. - PubMed
Hanauer 1996 {published data only}
-
- Hanauer S, Powers B, Robinson M, Mayle J, Elson C. Maintenance of remission of ulcerative colitis by mesalamine (Asacol) versus placebo. Gastroenterology 1994;106:A696.
-
- Hanauer SB, Sninsky CA, Robinson M, Powers BJ, McHattie JD, Mayle JE, et al. An oral preparation of mesalamine as long-term maintenance therapy for ulcerative colitis. A randomized, placebo-controlled trial. Annals of Internal Medicine 1996;124:204-11. - PubMed
Hawkey 1997 {published data only}
-
- Hawkey CJ, Dube LM, Rountree LV, Linnen PJ, Lancaster JF, the European Zileuton Study Group for Ulcerative Colitis. A trial of zileuton versus mesalazine or placebo in the maintenance of remission of ulcerative colitis. Gastroenterology 1997;112:718-24. - PubMed
Hawthorne 2012 {published data only}
-
- Hawthorne AB, Stenson R, Gillespie D, Swarbrick ET, Dhar A, Kapur KC, et al. Once daily asacol in maintenance therapy for ulcerative colitis: a one-year singe-blind randomised trial. Gut 2011;60:A37-8.
-
- Hawthorne AB, Stenson R, Gillespie D, Swarbrick ET, Dhar A, Kapur KC, et al. Once daily mesalazine as maintenance therapy for ulcerative colitis (UC): a one-year single-blind randomised trial. Gastroenterology 2011;140(5 Suppl 1):S65.
-
- Hawthorne AB, Stenson R, Gillespie D, Swarbrick ET, Dhar A, Kapur KC, et al. One-year investigator-blind randomized multicenter trial comparing Asacol 2.4 g once daily with 800 mg three times daily for maintenance of remission in ulcerative colitis. Inflammatory Bowel Disease 2012;18(10):1885-93. - PMC - PubMed
Ireland 1988a {published data only}
-
- Ireland A, Jewell DP. Comparative trial of olsalazine and sulfasalazine for the maintenance treatment of ulcerative colitis (UC). Gastroenterology 1987;92:A1447.
-
- Jewell DP, Ireland A. Controlled trial comparing olsalazine and sulfasalazine for maintenance treatment of ulcerative colitis. Scandinavian Journal of Gastroenterology. Supplement 1988;148:45-7. - PubMed
Ito 2010 {published data only}
Kamm 2008 {published data only}
-
- Kamm MA, Colombel J, Kornbluth A, Diebold R, Barrett K, Karlstadt RG, et al. A randomized comparison of once- versus twice-daily MMX® mesalamine for the maintenance of remission in mild-to-moderate ulcerative colitis. Gastroenterology 2007;132(4 Suppl 1):A510.
-
- Kamm MA, Schreiber S, Butler T, Barrett K, Stephenson D, Joseph RE. Safety analysis of MMX mesalazine for the maintenance of remission of mild-to-moderate ulcerative colitis: results of 4-month interim data analysis. In: UEGW Abstract Database. 2006:MON-G-230.
-
- Rubin D, Dubinsky M, Panaccione R, Siegel C, Binion D, Kane S, et al. Ulcerative colitis: patients’ perceptions compared with other chronic diseases. Inflammatory Bowel Diseases 2008;14(S1):S22.
-
- Sandborn W, Kamm M, Lichtenstein G, Barrett K, Joseph R. MMX™ mesalamine for the maintenance of remission in mild-to-moderate ulcerative colitis. Inflammatory Bowel Diseases 2008;14(S1):S24. - PubMed
Kane 2003b {published data only}
-
- Kane S, Huo D, Magnanti K. A pilot feasibility study of once daily versus conventional dosing mesalamine for maintenance of ulcerative colitis. Clinical Gastroenterology and Hepatology 2003;1(3):170-3. - PubMed
Kane 2008b {published data only}
Kiilerich 1992 {published data only}
-
- Kiilerich S, Ladefoged K, Rannem T, Ranlov P. Prophylactic effects of olsalazine (OLZ) versus sulfasalazine (SASP) in 12 months' maintenance treatment of ulcerative colitis. Gastroenterology 1991;100(5 Part 2):A220.
Kruis 1995 {published data only}
-
- Kruis W, Brandes JW, Schreiber S, Theuer D, Krakamp B, Schutz E. Olsalazine versus mesalazine in the treatment of mild to moderate ulcerative colitis. Alimentary Pharmacology & Therapeutics 1998;12(8):707-15. - PubMed
-
- Kruis W, Judmaier G, Kayasseh L, Stole M, Theuer D, Scheurlen C, et al. Double-blind dose-finding study of olsalazine versus sulphasalazine as maintenance therapy for ulcerative colitis. European Journal of Gastroenterology and Hepatology 1995;7:391-6. - PubMed
-
- Kruis W, Judmaier G, Kayasseh L, Stolte M, Scheurlen C, Hentschel E, et al. Double-blind dose-finding study of olsalazine vs. sulfasalazine for maintenance therapy of ulcerative colitis. Gastroenterology 1992;102:A649. - PubMed
-
- Kruis W, Stolte M. A double-blind dose finding study of olsalazine for the maintenance treatment of quiescent ulcerative colitis. Gastroenterology 1991;100(5 Part 2):A222. - PubMed
Kruis 2001 {published data only}
-
- Kruis W, Schreiber S, Theuer D, Brandes JW, Schutz E, Howaldt S, et al. Low dose balsalazide (1.5 g twice daily) and mesalazine (0.5 g three times daily) maintained remission of ulcerative colitis but high dose balsalazide (3.0 g twice daily) was superior in preventing relapses. Gut 2001;49(6):783-9. - PMC - PubMed
Kruis 2011 {published data only}
-
- Kruis W, Greinwald R, Mueller R. Optimal dosing of mesalamine for maintenance of clinical remission in patients with distal ulcerative colitis and residual endoscopic activity: a subgroup analysis of a double-blind, double-dummy, randomized, controlled, dose-ranging study. Gastroenterology 2013;144(5 Suppl 1):S768.
-
- Kruis W, Jonaitis L, Pokrotnieks J, Acute G, Mikhailova TL, Horynski M, et al. Once Daily 3g mesalamine is the optimal dose for maintaining clinical remission in ulcerative colitis: a double-blind, double-dummy, randomized, controlled, dose-ranging study. Gastroenterology 2008;134(4 Suppl 1):A489.
-
- Kruis W, Jonaitis L, Pokrotnieks J, Mikhailova TL, Horynski M, Bátovský M, et al. Randomised clinical trial: a comparative dose-finding study of three arms of dual release mesalazine for maintaining remission in ulcerative colitis. Alimentary Pharmacology and Therapeutics 2011;33(3):313-22. - PubMed
Lichtenstein 2010 {published data only}
-
- Lichtenstein GR, Gordon GL, Zakko S, Murthy U, Sedghi S, Pruitt R, et al. Clinical trial: once-daily mesalamine granules for maintenance of remission of ulcerative colitis – a 6-month placebo-controlled trial. Alimentary Pharmacology and Therapeutics 2010;32(8):990-9. - PubMed
Mahmud 2002 {published data only}
-
- Mahmud N, O'Toole D, O'Hare N, Freyne PJ, Weir DG, Kelleher D. Evaluation of renal function following treatment with 5-aminosalicylic acid derivatives in patients with ulcerative colitis. Alimentary Pharmacology and Therapeutics 2002;16(2):207-15. - PubMed
McIntyre 1988 {published data only}
-
- McIntyre PB, Rodrigues CA, Lennard-Jones JE, Barrison IG, Walker JG, Baron JH. Double blind controlled comparison of balsalazide and sulphasalazine in maintenance therapy of patients with ulcerative colitis [abstract]. Gut 1986;27:A1271. - PubMed
-
- McIntyre PB, Rodrigues CA, Lennard-Jones JE, Barrison IG, Walker JG, Baton JH, et al. Balsalazine in the maintenance treatment of patients with ulcerative colitis, a double-blind comparison with sulphasalazine. Alimentary Pharmacology and Therapeutics 1988;2:237-43. - PubMed
Miner 1995 {published data only}
-
- Miner P, Hanauer S, Robinson M, Schwartz J, Arora S, Pentasa UC Maintenance Study Group. Safety and efficacy of controlled-release mesalamine for maintenance of remission in ulcerative colitis. Digestive Diseases and Sciences 1995;40:296-304. - PubMed
-
- Miner P, Schwartz J, Aora S, Robinson M, Hanauer S, the Pentasa Study Group. Maintenance of remission in ulcerative colitis (UC) patients with controlled-release mesalamine capsules (Pentasa). Gastroenterology 1992;102:A666.
Mulder 1988 {published data only}
-
- Mulder CJ, Tytgat GN, Weterman IT, Dekker W, Blok P, Schrijver M, et al. Double-blind comparison of slow-release 5-aminosalicylate and sulfasalazine in remission maintenance in ulcerative colitis. Gastroenterology 1988;95:1449-53. - PubMed
-
- Mulder CJ, Tytgat GN, Weterman IT. Double blind evaluation of slow-release 5-aminosalicylic acid (Pentasa) and salazopyridin (Salazopyrin) in the maintenance therapy of ulcerative colitis. Gastroenterology 1988;94(5 Part 2):A313.
Nilsson 1995 {published data only}
-
- Nilsson A, Danielsson A, Lofberg R, Benno P, Bergman L, Fausa O, et al. Olsalazine versus sulphasalazine for relapse prevention in ulcerative colitis: a multicenter study. American Journal of Gastroenterology 1995;90:381-7. - PubMed
Paoluzi 2005 {published data only}
-
- Paoluzi OA, Iacopini F, Pica R, Crispino P, Marcheggiano A, Consolazio A, et al. Comparison of two different daily dosages (2.4 vs. 1.2 g) of oral mesalazine in maintenance of remission in ulcerative colitis patients: 1-year follow-up study. Alimentary Pharmacology and Therapeutics 2005;21(9):1111-9. - PubMed
-
- Paoluzi OA, Pica R, Crispino P, Iacopini F, Consolazio A, Rivera M, et al. Doubling daily dosage of oral mesalazine (2.4 vs. 1.2 g) in the maintenance therapy of pts with ulcerative colitis delays but does not reduce incidence of relapses: a 1-year follow-up study. Gastroenterology 2005;128(4 Suppl 2):A585.
Park 2019 {published data only}
-
- Park SK, Eun CS, Seo GS, Lim J, Kim TO, Park DI. The effects and adherence of Asacol comparing 2.4 g once daily with 800 mg three times or 1200 mg twice daily for maintain therapy in the ulcerative colitis: prospective multicentre randomised study. Journal of Crohn's & Colitis 2018;12(Suppl 1):S367.
Pica 2012 {published data only}
-
- Pica R, Cassieri C, Cocco A, Marcheggiano A, Occhigrossi G, Zippi M, et al. Randomised open label trial comparing two different dosages of oral mesalazine in the maintenance treatment of ulcerative colitis. Italian Journal of Medicine 2012;1:108.
Prantera 2009 {published data only}
-
- Kohn A, Prantera C, Caprilli R, Campieri M, Cottone M, Pallone F, et al. Maintenance treatment of ulcerative colitis with 5-aminosalicylic acid (5-ASA): results from the Italian population of a one year, randomized, multinational study comparing MMx® with Asacol®. Gastroenterology 2009;136(5 Suppl 1):390.
-
- Prantera C, Kohn A, Campieri M, Caprilli R, Cottone M, Pallone F, et al. Clinical trial: ulcerative colitis maintenance treatment with 5-ASA: a 1-year, randomized multicentre study comparing MMX with Asacol. Alimentary Pharmacology and Therapeutics 2009;30(9):908-18. - PubMed
Rijk 1992 {published data only}
-
- Rijk MC, Lier HJ, Tongerson JH. Relapse-preventing effect and safety of sulfasalazine and olsalazine in patients with ulcerative colitis in remission: prospective, double-blind, randomized multicenter study. American Journal of Gastroenterology 1992;87:438-42. - PubMed
-
- Rijk MC, Tongersen JH. The relapse preventing effect and safety of sulphasalazine and olsalazine in patients with ulcerative colitis in remission. Gastroenterology 1991;100(5 Part 2):A243.
Riley 1988 {published data only}
-
- Riley SA, Mani V, Goodman MJ, Herd ME, Dutt S, Turnberg LA. Comparison of delayed release 5 aminosalicylic acid (mesalazine) and sulfasalazine as maintenance treatment of patients with ulcerative colitis. Gastroenterology 2012;29(5):669-74. - PubMed
-
- Riley SA, Mani V, Goodman MJ, Herd ME, Dutt S, Turnberg LA. Comparison of delayed-release 5-aminosalicylic acid (mesalazine) and sulfasalazine as maintenance treatment for patients with ulcerative colitis. Gastroenterology 1988;94:1383-9. - PubMed
-
- Riley SA, Mani V, Goodman MJ, Turnberg LA. A comparison of delayed-release 5-ASA and sulfasalazine as maintenance treatment in ulcerative colitis. Gastroenterology 1987;92:A1596. - PubMed
-
- Riley SA, Mani V, Goodman MJ, Turnberg LA. A comparison of delayed-release 5-aminosalicylic acid (5-ASA) and sulfasalazine (SSZ) as maintenance treatment of ulcerative colitis (UC). Gut 1987;28:1390.
Rutgeerts 1989 {published data only}
-
- Rutgeerts P. Comparative efficacy of coated, oral 5-aminosalicylic acid (Claversal) and sulphasalazine for maintaining remission of ulcerative colitis. Alimentary Pharmacology and Therapeutics 1989;3:183-91. - PubMed
Sandberg‐Gertzen 1986 {published data only}
-
- Jarnerot G, Sandberg-Gertzen H. Azodisal sodium, (ADS), Dipentum, as relapse prevention in ulcerative colitis (UC). A double-blind placebo controlled study. Gastroenterology 1985;88(5 Part 2):A1432.
-
- Sandberg-Gertzen H, Jarnerot G, Kraaz W. Azodisal sodium in the treatment of ulcerative colitis: a study of tolerance and relapse prevention properties. Gastroenterology 1986;90:1024-30. - PubMed
Sandborn 2010 {published data only}
-
- Sandborn W, Kane S, Korzenik J, Lashner B, Leighton J, Mahadevan U, et al. Once daily dosing of delayed-release oral mesalamine for maintenance of remission of ulcerative colitis (the QDIEM trial): 6 and 12 month results. Inflammatory Bowel Diseases 2009;15:S15.
-
- Sandborn WJ, Korzenik J, Lashner B, Leighton JA, Mahadevan U, Marion JF, et al. Once-daily dosing of delayed-release oral mesalamine (400-mg tablet) is as effective as twice-daily dosing for maintenance of remission of ulcerative colitis. Gastroenterology 2010;138(4):1286-96. - PubMed
Suzuki 2017 {published data only}
-
- Suzuki Y, lida M, Ito H, Nishino H, Ohmori T, Arai T, et al. 2.4g Mesalamine (Asacol 400mg tablet) once daily is as effective as three times daily in maintenance of remission in ulcerative colitis: a randomized, noninferiority, multi-center trial. Inflammatory Bowel Diseases 2017;23(5):822-32. - PMC - PubMed
Travis 1994 {published data only}
Watanabe 2013 {published data only}
-
- Watanabe M, Hanai H, Nishino H, Yokoyama T, Terada T, Suzuki Y. Comparison of QD and TID oral mesalazine for maintenance of remission in quiescent ulcerative colitis: a double-blind, double-dummy, randomized multicenter study. Inflammatory Bowel Diseases 2013;19(8):1681-90. - PubMed
Wright 1993 {published data only}
-
- Wright JP, O'Keefe EA, Cuming L, Jaskiewicz K. Olsalazine in maintenance of clinical remission in patients with ulcerative colitis. Digestive Diseases and Sciences 1993;38:1837-42. - PubMed
-
- Wright JP, O'Keefe EA, Cuming L, Jaskiewicz K. Olsalazine in the maintenance of remission in patients with ulcerative colitis. Gastroenterology 1992;102:A714. - PubMed
References to studies excluded from this review
Andreoli 1994 {published data only}
-
- Andreoli A, Spinella S, Levenstein S, Prantera C. 5-ASA enema versus oral sulphasalazine in maintaining remission in ulcerative colitis. Italian Journal of Gastroenterology 1994;26(3):121-5. - PubMed
Bardazzi 1994 {published data only}
-
- Bardazzi G, d'Albasio G, Bonanomi AG, Trallori G, Messori A, Amorosi A, et al. Intermittent versus continuous 5-aminosalicylic acid treatment for maintaining remission in ulcerative colitis. Italian Journal of Gastroenterology 1994;26(7):334-7. - PubMed
d'Albasio 1997 {published data only}
-
- d'Albasio G, Pacini F, Camarri E, Messori A, Trallori G, Bonanomi AG, et al. Combined therapy with 5-aminosalicylic acid tablets and enemas for maintaining remission in ulcerative colitis: a randomized double-blind study. American Journal of Gastroenterology 1997;92(7):1143-7. - PubMed
D'Haens 2017 {published data only}
-
- D'Haens GR, Sandborn WJ, Zou G, Stitt LW, Rutgeerts PJ, Gilgen D, et al. Randomised non-inferiority trial: 1600 mg versus 400 mg tablets of mesalazine for the treatment of mild-to-moderate ulcerative colitis. Alimentary Pharmacology & Therapeutics 2017;46(3):292-302. - PubMed
Dew 1982b {published data only}
Dignass 2009b {published data only}
-
- Dignass A, Bokemeyer B, Stijnen T, Tan T, Borner N, Oudkerk Pool M, et al. Safety of once-daily vs twice-daily mesalazine (Pentasa) dosing. Results from a 12-month randomised controlled trial in maintenance of remission of ulcerative colitis. In: Canadian Journal of Gastroenterology. Vol. 23. 2009.
Dignass 2018 {published data only}
Eliakim 1990 {published data only}
-
- Eliakim R, Wengrower D, Ligumsky M, Rachmilewitz D. Comparable efficacy of oral 5-ASA (Mesasal) and sulfasalazine in maintaining ulcerative colitis in remission. Israel Journal of Medical Sciences 1990;26(1):47-9. - PubMed
Ewe 1996 {published data only}
-
- Ewe K, Becker K, Ueberschaer B. Systemic uptake of 5-aminosalicylic acid from olsalazine and Eudragit L coated mesalazine in patients with ulcerative colitis in remission. Zeitschrift fur Gastroenterologie 1996;34(4):225-9. - PubMed
Fernández‐Bañares 1999 {published data only}
-
- Fernández-Bañares F, Hinojosa J, Sánchez-Lombraña JL, Navarro E, Martínez-Salmerón JF, García-Pugés A, et al. Randomized clinical trial of Plantago ovata seeds (dietary fiber) as compared with mesalamine in maintaining remission in ulcerative colitis. American Journal of Gastroenterology 1999;94(2):427-33. - PubMed
Frieri 2005 {published data only}
-
- Frieri G, Pimpo M, Galletti B, Palumbo G, Corrao G, Latella G, et al. Long-term oral plus topical mesalazine in frequently relapsing ulcerative colitis. Digestive and Liver Disease 2005;37(2):92-6. - PubMed
Giaffer 1992b {published data only}
-
- Giaffer MH, O'Brien CJ, Holdsworth CD. Clinical tolerance to three 5-aminosalicylic acid releasing preparations in patients with inflammatory bowel disease intolerant or allergic to sulphasalazine. Alimentary Pharmacology and Therapeutics 1992;6(1):51-9. - PubMed
Gillespie 2014 {published data only}
-
- Gillespie D, Hood K, Farewell D, Stenson R, Probert C, Hawthorne AB. Electronic monitoring of medication adherence in a 1-year clinical study of 2 dosing regimens of mesalazine for adults in remission with ulcerative colitis. Inflammatory Bowel Diseases 2014;20(1):82-91. - PubMed
Gionchetti 1990 {published data only}
-
- Gionchetti P, Campieri M, Belluzzi A, Brignola C, Tampieri M, Iannone P, et al. Pentasa in maintenance treatment of ulcerative colitis. Gastroenterology 1990;98:251. - PubMed
Gionchetti 1996 {published data only}
-
- Gionchetti P, Campieri M, Venturi A, Rizzello F, Ferretti M, Brignola C, et al. Systemic availability of 5-aminosalicylic acid: comparison of delayed release and an azo-bond preparation. Alimentary Pharmacology and Therapeutics 1996;10(4):601-5. - PubMed
Green 2004 {published data only}
-
- Green JR, Swan CH, Gibson JA, Kerr GD, Swarbrick ET, Thornton PC. Patient-led variable dosing with balsalazide as long-term therapy for maintenance in ulcerative colitis: a 3-year prospective observational study. Alimentary Pharmacology and Therapeutics 2004;19(4):435-42. - PubMed
Hanauer 2009 {published data only}
Karamanolis 1996 {published data only}
-
- Karamanolis DG, Papatheodoridis GV, Xourgias V. Systemic absorption of 5-aminosalicylic acid in patients with inactive ulcerative colitis treated with olsalazine and mesalazine. European Journal of Gastroenterology and Hepatology 1996;8(11):1083-8. - PubMed
Kruis 1997 {published data only}
-
- Kruis W, Schütz E, Fric P, Fixa B, Judmaier G, Stolte M. Double-blind comparison of an oral Escherichia coli preparation and mesalazine in maintaining remission of ulcerative colitis. Alimentary Pharmacology and Therapeutics 1997;11(5):853-8. - PubMed
Kruis 2004 {published data only}
Levine 2017 {published data only}
-
- Levine A, Yerushalmi B, Kori M, Broide E, Mozer-Glassberg Y, Shaoul R, et al. Mesalamine enemas for induction of remission in pediatric ulcerative colitis refractory to oral mesalamine: a prospective cohort study. Journal of Crohn's & Colitis 2017;11(Suppl 1):S285. - PubMed
-
- Levine A, Yerushalmi B, Kori M, Broide E, Mozer-Glassberg Y, Shaoul R. Mesalamine enemas for induction of remission in pediatric ulcerative colitis refractory to oral Mesalamine: a prospective cohort study. Journal of Pediatric Gastroenterology and Nutrition 2017;64(Suppl 1):49. - PubMed
Mani 1994 {published data only}
-
- Mani V, Gotch P. Comparison of pharmacokinetics and colonic transit time in quiescent ulcerative colitis on mesalazine and olsalazine – a cross over study. Gut 1994;35(Suppl 4):A124.
Mantzaris 2004 {published data only}
-
- Mantzaris GJ, Sfakianakis M, Archavlis E, Petraki K, Christidou A, Karagiannidis A, et al. A prospective randomized observer-blind 2-year trial of azathioprine monotherapy versus azathioprine and olsalazine for the maintenance of remission of steroid-dependent ulcerative colitis. American Journal of Gastroenterology 2004;99(6):1122-8. - PubMed
Odes 1997 {published data only}
-
- Odes HS. 5-Aminosalicylic acid, 1,000-mg caplets versus 500-mg tablets, in maintenance of remission in ulcerative colitis. Journal of Clinical Gastroenterology 1997;24(4):287-8. - PubMed
Osterman 2014 {published data only}
Paoluzi 2002 {published data only}
-
- Paoluzi P, D'Albasio G, Pera A, Bianchi Porro G, Paoluzi OA, Pica R, et al. Oral and topical 5-aminosalicylic acid (mesalazine) in inducing and maintaining remission in mild-moderate relapse of ulcerative colitis: one-year randomised multicentre trial. Digestive and Liver Disease 2002;34(11):787-93. - PubMed
Papatheodoridis 1995 {published data only}
-
- Papatheodoridis GV, Xourgias V, Sdonas T, Triantoafyllou M, Tzouvaia M, Karamanolis DG. Systematic load of 5-ASA in patients with inactive ulcerative colitis treated with olsalazine and mesalazine. Gut 1995;37(Suppl 2 Pt 2):A54.
Pelech 1998 {published data only}
-
- Pelech T, Fric P, Fixa B, Komarkova O. Comparison of Mutaflor and mesalazine in the maintenance treatment of inactive ulcerative colitis. Prakticky Lekar 1998;78(10):556-8.
Pica 2015 {published data only}
-
- Pica R, Cassieri C, Cocco A, Zippi M, Marcheggiano A, De Nitto D, et al. A randomized trial comparing 4.8 vs. 2.4 g/day of oral mesalazine for maintenance of remission in ulcerative colitis. Digestive & Liver Disease 2015;47(11):933-7. - PubMed
Rubin 2017 {published data only}
Scherl 2013 {published data only}
-
- Scherl E, Pruitt R, Sedghi S, Barrett A, Bortey E, Paterson C, et al. Long-term safety and tolerability of twice-daily balsalazide disodium tablets in patients with ulcerative colitis. American Journal of Gastroenterology 2013;108:S545.
Staerk Laursen 1990 {published data only}
-
- Staerk Laursen L, Stokholm M, Bukhave K, Rask-Madsen J, Lauritsen K. Disposition of 5-aminosalicylic acid by olsalazine and three mesalazine preparations in patients with ulcerative colitis: comparison of intraluminal colonic concentrations, serum values, and urinary excretion. Gut 1990;31:1271-6. - PMC - PubMed
Stoa‐Birketvedt 1999 {published data only}
-
- Florholmen J. The systemic load and efficient delivery of active 5-ASA in patients with ulcerative colitis on treatment with Dipentum (olsalazine) and Mesasael (mesalazine). Gut 1994;35(Suppl 4):A121. - PubMed
-
- Stoa-Birketvedt G, Florholmen J. The systemic load and efficient delivery of active 5-aminosalicylic acid in patients with ulcerative colitis on treatment with olsalazine or mesalazine. Alimentary Pharmacology and Therapeutics 1999;13:357-61. - PubMed
Sun 2016 {published data only}
-
- Sun J, Yuan Y. Mesalazine modified-release tablet in the treatment of ulcerative colitis in the remission phase: a Chinese, multicenter, single-blind, randomized controlled study. Advances in Therapy 2016;33(3):410-22. - PubMed
Tragnone 1996 {published data only}
-
- Tragnone A, Elmi G, Tagliente C, Bazzocchi G, Dina R, Euseb V, et al. Oral 5-aminosalicylic acid therapy for the prevention of relapse in patients with ulcerative colitis (UC) during remission: a new therapeutical approach by using 5-ASA tablets 800 mg. A multicenter controlled randomized trial. Gastroenterology 1996;110:A1030.
Turner 2017 {published data only}
-
- Turner D, Yerushalmi B, Kori M, Broide E, Mozer-Glassberg Y, Shaoul R, et al. Once- versus twice-daily mesalazine to induce remission in paediatric ulcerative colitis: a randomised controlled trial. Journal of Crohn's & Colitis 2017;11(5):527-33. - PubMed
Tzivras 1997 {published data only}
-
- Archimandritis A, Hatzis G, Konstandinidis A, Paraskeva K, Tjivras M, Skandalis N. Disposition of 5-ASA by olsalazine (Dipentum) and mesalazine (Asacol) in patients with ulcerative colitis in remission. Gut 1996;39(Suppl 3):A75.
-
- Tzivras M, Konstandinidis A, Hatzis G, Paraskeva K, Skandalis N, Archimandritis A. Systemic absorption of 5-aminosalicylic acid in patients with inactive ulcerative colitis treated with olsalazine and mesalazine. European Journal of Gastroenterology and Hepatology 1997;9(7):729-30. - PubMed
Yokoyama 2007 {published data only}
-
- Yokoyama H, Takagi S, Kuriyama S, Takahashi S, Takahashi H, Iwabuchi M, et al. Effect of weekend 5-aminosalicylic acid (mesalazine) enema as maintenance therapy for ulcerative colitis: results from a randomized controlled study. Inflammatory Bowel Diseases 2007;13(9):1115-20. - PubMed
Zocco 2006 {published data only}
-
- Zocco MA, Dal Verme LZ, Cremonini F, Piscaglia AC, Nista EC, Candelli M, et al. Efficacy of Lactobacillus GG in maintaining remission of ulcerative colitis. Alimentary Pharmacology and Therapeutics 2006;23:1567-74. - PubMed
References to ongoing studies
NCT02280629 {published data only}
-
- NCT02280629. Phosphatidylcholine (LT-02) vs. placebo vs. mesalamine for maintenance of remission in ulcerative colitis (PROTECT-2) [Randomized, double-blind, double-dummy, placebo-controlled, phase III clinical trial on the efficacy and safety of a 48-weeks treatment with gastro-resistant phosphatidylcholine (LT-02) versus placebo versus mesalamine for maintenance of remission in patients with ulcerative colitis]. clinicaltrials.gov/show/NCT02280629 (first received 31 October 2014).
NCT02522780 {published data only}
-
- NCT02522780. Mesalamine 2 g Sachet for the maintenance of clinical and endoscopic remission in ulcerative colitis (UC) [A randomized, double-blind, placebo-controlled, multicenter study investigating the efficacy and safety of mesalamine 2 g extended release granules (Sachet) for maintenance of clinical and endoscopic remission in ulcerative colitis]. clinicaltrials.gov/show/NCT02522780 (first received 13 August 2015).
Additional references
Andrade 1995
-
- Andrade SE, Walker AM, Gottlieb LK, Hollenberg NK, Testa MA, Saperia GM, et al. Discontinuation of antihyperlipidemic drugs – do rates reported in clinical trials reflect rates in primary care settings? New England Journal of Medicine 1995;332(17):1125-31. - PubMed
Azad Khan 1977
-
- Azad Khan AK, Piris J, Truelove SC. An experiment to determine the active therapeutic moiety of sulfasalazine. Lancet 1977;2(8044):892-5. - PubMed
Beaulieu 2009
-
- Beaulieu DB Schwartz DA. Medication persistence in patients with ulcerative colitis: meeting the challenges and improving patient outcomes CME. MedScape CME Gastroenterology Posted 22 December 2009.
Brixner 2007
-
- Brixner D, Magowan S, Accortt N. Evaluation of prescription refill patterns based on daily dosing regimen and pill load for calcium channel blockers. Academy of Managed Care Pharmacy 19th Annual Meeting; 2007 Apr 10–13; San Diego (CA), 2007.
Chan 1983
-
- Chan RP, Pope DJ, Gilbert AP, Sacra PJ, Baron JH, Lennard-Jones JE. Studies of two novel sulfasalazine analogs, ipsalazide and balsalazide. Digestive Diseases and Sciences 1983;28:609-15. - PubMed
Danese 2011
-
- Danese S, Fiocchi C. Ulcerative colitis. New England Journal of Medicine 2011;365(18):1713-25. - PubMed
Das 1973
-
- Das KM, Eastwood MA, McManus JP, Sircus W. Adverse reactions during salicylazosulfapyridine therapy and the relation with drug metabolism and acetylator phenotype. New England Journal of Medicine 1973;289:491-5. - PubMed
Dew 1982a
Ediger 2007
-
- Ediger JP, Walker JR, Graff L, Lix L, Clara I, Rawsthorne P, et al. Predictors of medication adherence in inflammatory bowel disease. American Journal of Gastroenterology 2007;102(7):1417-26. - PubMed
Feuerstein 2014
-
- Feuerstein JD, Cheifetz AS. Ulcerative colitis. Mayo Clinic Proceedings 2014;89(11):1553-63. - PubMed
Fleig 1988
-
- Fleig WE, Laudage G, Sommer H, Wellmann W, Stange EF, Riemann J. Prospective, randomized, double-blind comparison of benzalazine and sulfasalazine in the treatment of active ulcerative colitis. Digestion 1988;40(3):173-80. - PubMed
Ford 2011a
-
- Ford AC, Achkar JP, Khan KJ, Kane SV, Talley NJ, Marshall JK, et al. Efficacy of 5-aminosalicylates in ulcerative colitis: systematic review and meta-analysis. American Journal of Gastroenterology 2011;106:601-16. - PubMed
Ford 2011b
-
- Ford AC, Khan KJ, Sandborn WJ, Kane SV, Moayyedi P. Once-daily dosing vs. conventional dosing schedule of mesalamine and relapse of quiescent ulcerative colitis: systematic review and meta-analysis. American Journal of Gastroenterology 2011;106:2070-7. - PubMed
Greenfield 1993
-
- Greenfield SM, Punchard NA, Teare JP, Thompson RP. Review article: the mode of action of the aminosalicylates in inflammatory bowel disease. Alimentary Pharmacology and Therapeutics 1993;7:369-83. - PubMed
Guyatt 2008
Hardy 1987
-
- Hardy JG, Healey JN, Reynolds JR. Evaluation of an enteric-coated delayed-release 5-aminosalicylic acid tablet in patients with inflammatory bowel disease. Alimentary Pharmacology and Therapeutics 1987;1:273-80. - PubMed
Higgins 2003
Higgins 2011a
-
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
-
- Higgins JP, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Ireland 1988b
-
- Ireland A, Jewell DP. Olsalazine in patients intolerant of sulfasalazine. Scandinavian Journal of Gastroenterology 1988;23(Suppl 148):101-3. - PubMed
Jarnerot 1996
-
- Jarnerot G. Withdrawal rates because of diarrhoea in Dipentum-treated patients with ulcerative colitis are low when Dipentum is taken with food and dose titrated [abstract]. Gastroenterology 1996;110:A932.
Kane 2001
-
- Kane SV, Cohen RD, Aikens JE, Hanauer SB. Prevalence of nonadherence with maintenance mesalamine in quiescent ulcerative colitis. American Journal of Gastroenterology 2001;96(10):2929-33. - PubMed
Kane 2003a
-
- Kane S, Huo D, Aikens J, Hanauer S. Medication nonadherence and the outcomes of patients with quiescent ulcerative colitis. American Journal of Medicine 2003;114(1):39-43. - PubMed
Kane 2006
-
- Kane SV. Systematic review: adherence issues in the treatment of ulcerative colitis. Alimentary Pharmacology and Therapeutics 2006;23(5):577-85. - PubMed
Kane 2008a
Kjaergaard 1989
-
- Kjaergaard N, Ambrosius Christensen L, Lauritsen JG, Rasmussen N, Honore Hansen S. Effects of mesalazine substitution on salicylazosulfapyridine-induced seminal abnormalities in men with ulcerative colitis. Scandinavian Journal of Gastroenterology 1989;24:891-6. - PubMed
Klotz 1980
-
- Klotz U, Maier K, Fischer C, Heinkel K. Therapeutic efficacy of sulfasalazine and its metabolites in patients with ulcerative colitis and Crohn's disease. New England Journal of Medicine 1980;303:1499-502. - PubMed
Levy 1999
-
- Levy RL, Feld AD. Increasing patient adherence to gastroenterology treatment and prevention regimens. American Journal of Gastroenterology 1999;94(7):1733-42. - PubMed
Loftus 2004
-
- Loftus EJ. Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology 2004;126(6):1504-17. - PubMed
Magowan 2006
-
- Magowan S, Kane S, Lange JL. 5-ASA prescription refill rates for ulcerative colitis are independent of formulation and dosing regimens. American Journal of Gastroenterology 2006;101:S447.
Misiewitz 1965
-
- Misiewitz JJ, Lennard-Jones JE, Connell AM, Baron JH, Jones FA. Controlled trial of sulfasalazine in maintenance therapy for ulcerative colitis. Lancet 1965;1:185-8.
Myers 1987
Nielsen 1982
-
- Nielsen OH. Sulfasalazine intolerance: a retrospective study of the reasons for discontinuing treatment with sulfasalazine in patients with chronic inflammatory bowel disease. Scandinavian Journal of Gastroenterology 1982;17:389-93. - PubMed
Nielsen 1983
Peppercorn 1972
-
- Peppercorn MA, Goldman P. The role of intestinal bacteria in the metabolism of salicylazo-sulfapyridine. Journal of Pharmacology and Experimental Therapeutics 1972;181:555-62. - PubMed
Rao 1987
-
- Rao SS, Cann PA, Holdsworth CD. Clinical experience of the tolerance of mesalazine and olsalazine in patients intolerant of sulfasalazine. Scandinavian Journal of Gastroenterology 1987;22:332-6. - PubMed
Rasmussen 1982
-
- Rasmussen SN, Bondesen S, Hvidberg EF, Hansen SH, Binder V, Halskou S, et al. 5-aminosalicylic acid in a slow-release preparation: bioavailability, plasma level, and excretion in humans. Gastroenterology 1982;83:1062-70. - PubMed
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riley 1987
Rothfuss 2006
Sandborn 2002a
-
- Sandborn WJ. Rational selection of oral 5-aminosalicylate formulations and prodrugs for the treatment of ulcerative colitis. American Journal of Gastroenterology 2002;97(12):2939-41. - PubMed
Sandborn 2002b
-
- Sandborn WJ, Hanauer SB, Buch A. Comparable systemic absorption of 5-ASA and N-AC-5-ASA from U.S. Asacol and Colazal. American Journal of Gastroenterology 2002;97:S263.
Sandborn 2002c
-
- Sandborn WJ, Hanauer SB. The pharmacokinetic profiles of oral 5ASA formulations used in the management of ulcerative colitis: a systematic review. American Journal of Gastroenterology 2002;97:S269.
Sandborn 2003
-
- Sandborn WJ, Hanauer SB. Systematic review: the pharmacokinetic profiles of oral mesalazine formulations and mesalazine pro-drugs used in the management of ulcerative colitis. Alimentary Pharmacology and Therapeutics 2003;17(1):29-42. - PubMed
Schroeder 1972
-
- Schroeder H, Campbell DE. Absorption, metabolism, and excretion of salicylazosulfapyridine in man. Clinical Pharmacology and Therapeutics 1972;13:539-51. - PubMed
Schünemann 2011
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Shale 2003
-
- Shale MJ, Riley SA. Studies of compliance with delayed-release mesalazine therapy in patients with inflammatory bowel disease. Alimentary Pharmacology and Therapeutics 2003;18(2):191-8. - PubMed
Svartz 1942
-
- Svartz N. Salazopyrin, a new sulfanilamide preparation: A. Therapeutic results in rheumatic polyarthritis. B. Therapeutic results in ulcerative colitis. C. Toxic manifestations in treatment with sulfanilamide preparation. Acta Medica Scandinavica 1942;110:557-90.
Truelove 1955
Truelove 1959
Van Hees 1980
References to other published versions of this review
Feagan 2012
Sutherland 1993
-
- Sutherland LR, May GR, Shaffer EA. Sulfasalazine revisited: a meta-analysis of 5-aminosalicylic acid in the treatment of ulcerative colitis. Annals of Internal Medicine 1993;118:540-9. - PubMed
Sutherland 1997
-
- Sutherland LR, Roth DE, Beck PL. Alternatives to sulfasalazine: A meta-analysis of 5-ASA in the treatment of ulcerative colitis. Inflammatory Bowel Diseases 1997;3:65-78. - PubMed
Sutherland 2006
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

