Effects of metronidazole on the fecal microbiome and metabolome in healthy dogs
- PMID: 32856349
- PMCID: PMC7517498
- DOI: 10.1111/jvim.15871
Effects of metronidazole on the fecal microbiome and metabolome in healthy dogs
Abstract
Background: Metronidazole has a substantial impact on the gut microbiome. However, the recovery of the microbiome after discontinuation of administration, and the metabolic consequences of such alterations have not been investigated to date.
Objectives: To describe the impact of 14-day metronidazole administration, alone or in combination with a hydrolyzed protein diet, on fecal microbiome, metabolome, bile acids (BAs), and lactate production, and on serum metabolome in healthy dogs.
Animals: Twenty-four healthy pet dogs.
Methods: Prospective, nonrandomized controlled study. Dogs fed various commercial diets were divided in 3 groups: control group (no intervention, G1); group receiving hydrolyzed protein diet, followed by metronidazole administration (G2); and group receiving metronidazole only (G3). Microbiome composition was evaluated with sequencing of 16S rRNA genes and quantitative polymerase chain reaction (qPCR)-based dysbiosis index. Untargeted metabolomics analysis of fecal and serum samples was performed, followed by targeted assays for fecal BAs and lactate.
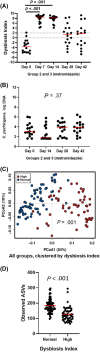
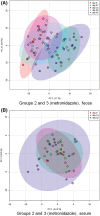
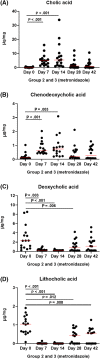
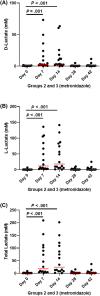
Results: No changes were observed in G1, or G2 during diet change. Metronidazole significantly changed microbiome composition in G2 and G3, including decreases in richness (P < .001) and in key bacteria such as Fusobacteria (q < 0.001) that did not fully resolve 4 weeks after metronidazole discontinuation. Fecal dysbiosis index was significantly increased (P < .001). Those changes were accompanied by increased fecal total lactate (P < .001), and decreased secondary BAs deoxycholic acid and lithocholic acid (P < .001).
Conclusion and clinical importance: Our results indicate a minimum 4-week effect of metronidazole on fecal microbiome and metabolome, supporting a cautious approach to prescription of metronidazole in dogs.
Keywords: antibiotic; bile acid metabolism; dysbiosis; fecal metabolome; microbiota; serum metabolome.
© 2020 The Authors. Journal of Veterinary Internal Medicine published by Wiley Periodicals LLC on behalf of American College of Veterinary Internal Medicine.
Conflict of interest statement
Rachel Pilla, Amanda B. Blake, Mohammad R. Khattab, Jonathan A. Lidbury, Jörg M. Steiner, and Jan S. Suchodolski are employed by the Gastrointestinal Laboratory at Texas A&M University, which provides assay for intestinal function and microbiota analysis on a fee‐for‐service basis. Frederic P. Gaschen, James W. Barr, Erin Olson, Julia Honneffer, Blake C. Guard, Dean Villanueva, and Mustafa K. AlShawaqfeh have no conflicts to declare.
Figures



References
-
- Machiels K, Joossens M, Sabino J, et al. A decrease of the butyrate‐producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut. 2014;63:1275‐1283. - PubMed
-
- Duboc H, Rajca S, Rainteau D, et al. Connecting dysbiosis, bile‐acid dysmetabolism and gut inflammation in inflammatory bowel diseases. Gut. 2013;62:531‐539. - PubMed
-
- Pavlidis P, Powell N, Vincent RP, Ehrlich D, Bjarnason I, Hayee B. Systematic review: bile acids and intestinal inflammation‐luminal aggressors or regulators of mucosal defence? Aliment Pharmacol Ther. 2015;42:802‐817. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

