Overall Survival of Nonmetastasized NSCLC Patients Treated With Add-On Viscum album L: A Multicenter Real-World Study
- PMID: 32856476
- PMCID: PMC7457695
- DOI: 10.1177/1534735420940384
Overall Survival of Nonmetastasized NSCLC Patients Treated With Add-On Viscum album L: A Multicenter Real-World Study
Abstract
Background: Recent data suggest a beneficial effect of add-on treatment with Viscum album L (VA) on the survival in cancer patients. The objective of this study was to compare the impact of standard oncological therapy plus add-on VA treatment (S+VA) versus standard oncological therapy alone (S) on the overall survival (OS) of patients with nonmetastasized non-small cell lung carcinoma (NSCLC).
Methods: The multicenter real-world data study was conducted using data from the Network Oncology Clinical Registry. The primary end point was OS. OS and impact on hazard in both treatment groups were compared.
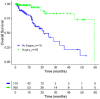
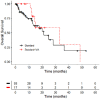
Results: A total of 275 patients with stages I to IIIA NSCLC were enrolled (mean age = 67.6 years, 57.2% male patients). No significant difference of OS was observed between both groups. Even though not significant, for a subgroup of unresected patients with stage I NSCLC, adenocarcinoma or squamous cell carcinoma, a medium effect size OS improvement was observed for S+VA compared to S.
Conclusions: Our findings support the importance of surgery as the most effective intervention in nonmetastasized NSCLC patients. Add-on VA therapy shows here no additional effect in resected patients. However, a small subgroup analysis suggests a possible role of add-on VA for nonresected subgroups. Our results complement existing knowledge on the clinical impact of add-on VA therapy in NSCLC patients and may serve as hypothesis-generating data for further examinations in this cohort. Further research could be directed towards the role of combined therapy for nonresected early-stage NSCLC.
Keywords: NSCLC; Viscum album L; advanced; lung cancer; nonmetastasized; overall survival.
Conflict of interest statement
Figures


References
-
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30. - PubMed
-
- EMA. Keytruda, INN-pembrolizumab. Annex I: summary of product characteristics. Accessed November 27, 2017 http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Info...
-
- Gettinger S, Horn L, Jackman D, et al. Five-year follow-up of nivolumab in previously treated advanced non-small-cell lung cancer: results from the CA209-003 study. J Clin Oncol. 2018;36:1675-1684. - PubMed
-
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

