Deletion of Microfibrillar-Associated Protein 4 Attenuates Left Ventricular Remodeling and Dysfunction in Heart Failure
- PMID: 32856514
- PMCID: PMC7660778
- DOI: 10.1161/JAHA.119.015307
Deletion of Microfibrillar-Associated Protein 4 Attenuates Left Ventricular Remodeling and Dysfunction in Heart Failure
Abstract
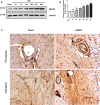
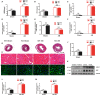
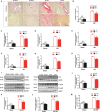
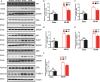
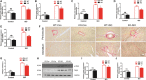
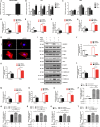
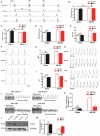
Background Cardiac remodeling predisposes individuals to heart failure if the burden is not solved, and heart failure is a growing cause of morbidity and mortality worldwide. The cardiac extracellular matrix not only provides structural support, but also is a core aspect of the myocardial response to various biomechanical stresses and heart failure. MFAP4 (microfibrillar-associated protein 4) is an integrin ligand located in the extracellular matrix, whose biological functions in the heart remain poorly understood. In the current study we aimed to test the role of MFAP4 in cardiac remodeling. Methods and Results MFAP4-deficient (MFAP4-/-) and wild-type mice were subjected to aortic banding surgery and isoproterenol to establish models of cardiac remodeling. We also evaluated the functional effects of MFAP4 on cardiac hypertrophy, fibrosis, and cardiac electrical remodeling. The expression of MFAP4 was increased in the animal cardiac remodeling models induced by pressure overload and isoproterenol. After challenge of 8 weeks of aortic banding or 2 weeks of intraperitoneal isoproterenol, MFAP4-/- mice exhibited lower levels of cardiac fibrosis and fewer ventricular arrhythmias than wild-type mice. However, there was no significant effect on cardiomyocyte hypertrophy. In addition, there was no significant difference in cardiac fibrosis severity, hypertrophy, or ventricular arrhythmia incidence between wild-type-sham and knockout-sham mice. Conclusions These findings are the first to demonstrate that MFAP4 deficiency inhibits cardiac fibrosis and ventricular arrhythmias after challenge with 8 weeks of aortic banding or 2 weeks of intraperitoneal isoproterenol but does not significantly affect the hypertrophy response. In addition, MFAP4 deficiency had no significant effect on cardiac fibrosis, hypertrophy, or ventricular arrhythmia in the sham group in this study.
Keywords: cardiac remodeling; extracellular matrix proteins; heart failure; microfibrillar-associated protein 4; pressure overload.
Conflict of interest statement
None.
Figures


References
-
- Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling–concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an international forum on cardiac remodeling. J Am Coll Cardiol. 2000;35:569–582. - PubMed
-
- Misra A, Mann DL. Treatment of heart failure beyond practice guidelines. Role of cardiac remodeling. Circ J. 2008;72(suppl A):A1–A7. - PubMed
-
- Aimo A, Gaggin HK, Barison A, Emdin M, Januzzi JL Jr. Imaging, biomarker, and clinical predictors of cardiac remodeling in heart failure with reduced ejection fraction. JACC Heart Fail. 2019;7:782–794. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

