Clinical Implications of Physical Function and Resilience in Patients Undergoing Transcatheter Aortic Valve Replacement
- PMID: 32856530
- PMCID: PMC7660783
- DOI: 10.1161/JAHA.120.017075
Clinical Implications of Physical Function and Resilience in Patients Undergoing Transcatheter Aortic Valve Replacement
Abstract
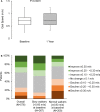
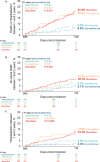
Background Gait speed is a reliable measure of physical function and frailty in patients with aortic stenosis undergoing transcatheter aortic valve replacement (TAVR). Slow gait speed pre-TAVR predicts worse clinical outcomes post-TAVR. The consequences of improved versus worsened physical function post-TAVR are unknown. Methods and Results The REPRISE III (Repositionable Percutaneous Replacement of Stenotic Aortic Valve Through Implantation of Lotus Valve System-Randomized Clinical Evaluation) trial randomized high/extreme risk patients to receive a mechanically-expanded or self-expanding transcatheter heart valve. Of 874 patients who underwent TAVR, 576 with complete data at baseline and 1 year were included in this analysis. Slow gait speed in the 5-m walk test was defined as <0.83 m/s. A clinically meaningful improvement (≥0.1 m/s) in gait speed 1 year after TAVR occurred in 39% of patients, 35% exhibited no change, and 26% declined (≥0.1 m/s). Among groups defined by baseline/1-year post-TAVR gait speeds, 1- to 2-year mortality or hospitalization rates were as follows: 6.6% (normal/normal), 8.0% (slow/normal), 20.9% (normal/slow), and 21.5% (slow/slow). After adjustment, slow gait speed at 1 year (regardless of baseline speed) was associated with a 3.5-fold increase in death/hospitalization between 1 and 2 years compared with those with normal baseline/1-year gait speed. Patients whose slow gait speed normalized at 1 year had no increased risk. One-year, but not baseline, gait speed was associated with death or hospitalization between 1 and 2 years (adjusted hazard ratio, 0.83 per 0.1 m/s faster gait; 95% CI, 0.74-0.93, P=0.001). Conclusions Marked heterogeneity exists in the trajectory of physical function after TAVR and this, more than baseline function, has clinical consequences. Identifying and optimizing factors associated with physical resilience after TAVR may improve outcomes. Registration URL: https://www.clinicaltrials.gov; Unique identifier: NCT02202434.
Keywords: aortic valve stenosis; frailty; gait speed; outcomes; physical function; transcatheter aortic valve replacement.
Conflict of interest statement
Dr Barker is on the medical advisory board for Boston Scientific. Dr Rajagopal is on the scientific advisory board for Boston Scientific and is a speaker/proctor for Medtronic, Edwards, and Abbott vascular; Dr Makkar receives research grants from Boston Scientific and Medtronic. Dr Bajwa is a consultant for Medtronic. Dr Kleiman provides educational services for Medtronic. Dr Linke has received speaker honoraria or served as a consultant for the following companies: Medtronic Inc., St. Jude Medical Inc., Claret Medical Inc., Boston Scientific, Edwards Lifesciences, Symetis as well as Bard, and owns stock option from Claret Medical Inc and receives grants and consultant fees from Medtronic, Edwards, Boston Scientific, and Abbott. Dr Kereiakes is a consultant and is on the Scientific Advisory Board, Boston Scientific, Inc and receives research grants from Edwards Lifesciences. Dr Waksman receives grant/research support or consulting fees/honoraria from Boston Scientific, Biotronik Biosensors, Medtronic Vascular, Abbott Vascular, Symetis, Med Alliance, LifeTech, Amgen, and Volcano/Philips. Dr Allocco is a full‐time employee and shareholder of Boston Scientific. Dr Reardon receives research grants from Boston Scientific and Medtronic. Dr Lindman has served on the scientific advisory board for Roche Diagnostics, has received research grants from Edwards Lifesciences and Roche Diagnostics, and has consulted for Medtronic. The remaining authors have no disclosures to report.
Figures
References
-
- Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, et al. Transcatheter versus surgical aortic‐valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–2198. - PubMed
-
- Adams DH, Popma JJ, Reardon MJ, Yakubov SJ, Coselli JS, Deeb GM, Gleason TG, Buchbinder M, Hermiller J Jr, Kleiman NS, et al. Transcatheter aortic‐valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;370:1790–1798. - PubMed
-
- Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, et al. Transcatheter or surgical aortic‐valve replacement in intermediate-risk patients. N Engl J Med. 2016;374:1609–1620. - PubMed
-
- Reardon MJ, Van Mieghem NM, Popma JJ, Kleiman NS, Sondergaard L, Mumtaz M, Adams DH, Deeb GM, Maini B, Gada H, et al. Surgical or transcatheter aortic‐valve replacement in intermediate-risk patients. N Engl J Med. 2017;376:1321–1331. - PubMed
-
- Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, Kapadia SR, Malaisrie SC, Cohen DJ, Pibarot P, et al. Transcatheter aortic‐valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380:1695–1705. - PubMed

