Intrapulmonary Pharmacokinetics of First-line Anti-tuberculosis Drugs in Malawian Patients With Tuberculosis
- PMID: 32856694
- PMCID: PMC8563277
- DOI: 10.1093/cid/ciaa1265
Intrapulmonary Pharmacokinetics of First-line Anti-tuberculosis Drugs in Malawian Patients With Tuberculosis
Abstract
Background: Further work is required to understand the intrapulmonary pharmacokinetics of first-line anti-tuberculosis drugs. This study aimed to describe the plasma and intrapulmonary pharmacokinetics of rifampicin, isoniazid, pyrazinamide, and ethambutol, and explore relationships with clinical treatment outcomes in patients with pulmonary tuberculosis.
Methods: Malawian adults with a first presentation of microbiologically confirmed pulmonary tuberculosis received standard 6-month first-line therapy. Plasma and intrapulmonary samples were collected 8 and 16 weeks into treatment and drug concentrations measured in plasma, lung/airway epithelial lining fluid (ELF), and alveolar cells. Population pharmacokinetic modeling generated estimates of drug exposure (Cmax and AUC) from individual-level post hoc Bayesian estimates of plasma and intrapulmonary pharmacokinetics.
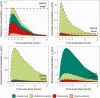
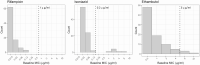
Results: One-hundred fifty-seven patients (58% HIV coinfected) participated. Despite standard weight-based dosing, peak plasma concentrations of first-line drugs were below therapeutic drug-monitoring targets. Rifampicin concentrations were low in all 3 compartments. Isoniazid, pyrazinamide, and ethambutol achieved higher concentrations in ELF and alveolar cells than plasma. Isoniazid and pyrazinamide concentrations were 14.6-fold (95% CI, 11.2-18.0-fold) and 49.8-fold (95% CI, 34.2-65.3-fold) higher in ELF than plasma, respectively. Ethambutol concentrations were highest in alveolar cells (alveolar cell-plasma ratio, 15.0; 95% CI, 11.4-18.6). Plasma or intrapulmonary pharmacokinetics did not predict clinical treatment response.
Conclusions: We report differential drug concentrations between plasma and the lung. While plasma concentrations were below therapeutic monitoring targets, accumulation of drugs at the site of disease may explain the success of the first-line regimen. The low rifampicin concentrations observed in all compartments lend strong support for ongoing clinical trials of high-dose rifampicin regimens.
Keywords: antitubercular; pharmacodynamics; pharmacokinetics; pulmonary; tuberculosis.
© The Author(s) 2020. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures
Comment in
-
The Relationship Between Drug Concentration in Tuberculosis Lesions, Epithelial Lining Fluid, and Clinical Outcomes.Clin Infect Dis. 2021 Nov 2;73(9):e3374-e3376. doi: 10.1093/cid/ciaa1271. Clin Infect Dis. 2021. PMID: 32857152 No abstract available.
References
-
- World Health Organization. Global tuberculosis report 2019. Geneva, Switzerland: World Health Organization, 2019.
-
- Wilkins JJ, Langdon G, McIlleron H, Pillai GC, Smith PJ, Simonsson US. Variability in the population pharmacokinetics of pyrazinamide in South African tuberculosis patients. Eur J Clin Pharmacol 2006; 62:727–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

