Clinical sensitivity and interpretation of PCR and serological COVID-19 diagnostics for patients presenting to the hospital
- PMID: 32856766
- PMCID: PMC7461169
- DOI: 10.1096/fj.202001700RR
Clinical sensitivity and interpretation of PCR and serological COVID-19 diagnostics for patients presenting to the hospital
Abstract
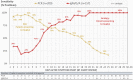
The diagnosis of COVID-19 requires integration of clinical and laboratory data. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) diagnostic assays play a central role in diagnosis and have fixed technical performance metrics. Interpretation becomes challenging because the clinical sensitivity changes as the virus clears and the immune response emerges. Our goal was to examine the clinical sensitivity of two most common SARS-CoV-2 diagnostic test modalities, polymerase chain reaction (PCR) and serology, over the disease course to provide insight into their clinical interpretation in patients presenting to the hospital. We conducted a single-center, retrospective study. To derive clinical sensitivity of PCR, we identified 209 PCR-positive SARS-CoV-2 patients with multiple PCR test results (624 total PCR tests) and calculated daily sensitivity from date of symptom onset or first positive test. Clinical sensitivity of PCR decreased with days post symptom onset with >90% clinical sensitivity during the first 5 days after symptom onset, 70%-71% from Days 9 to 11, and 30% at Day 21. To calculate daily clinical sensitivity by serology, we utilized 157 PCR-positive patients with a total of 197 specimens tested by enzyme-linked immunosorbent assay for IgM, IgG, and IgA anti-SARS-CoV-2 antibodies. In contrast to PCR, serological sensitivity increased with days post symptom onset with >50% of patients seropositive by at least one antibody isotype after Day 7, >80% after Day 12, and 100% by Day 21. Taken together, PCR and serology are complimentary modalities that require time-dependent interpretation. Superimposition of sensitivities over time indicate that serology can function as a reliable diagnostic aid indicating recent or prior infection.
Keywords: COVID; SARS-CoV-2; biomarker; qPCR.
© 2020 Massachusetts General Hospital, Center for Integrated Diagnostics. The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Conflict of interest statement
Dr Gelfand reports personal fees from Henry Schein Inc, outside the submitted work; Dr Turbett reports grants from Centers for Disease Control and Prevention, outside the submitted work; Dr Anahtar reports personal fees and other from Day Zero Diagnostics, outside the submitted work; Dr Ryan reports grants from CDC, during the conduct of the study. Dr. Branda reports grants from Zeus Scientific, grants from bioMerieux, grants from Immunetics, personal fees from T2 Biosystems, personal fees from DiaSorin, personal fees from Roche Diagnostics, grants from Bay Area Lyme Foundation, grants from Lyme Disease Biobank Foundation, outside the submitted work.
Figures
References
-
- Sethuraman N, Jeremiah SS, Ryo A. Interpreting diagnostic tests for SARS‐CoV‐2. JAMA. 2020;323(22):2249. - PubMed
-
- Wolfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID‐2019. Nature. 2020;581(7809):465‐469. - PubMed
-
- He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID‐19. Nat Med. 2020;26(5):672‐675. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

