Description of Chiral Complexes within Functional-Group Symmetry-Adapted Perturbation Theory-The Case of (S/R)-Carvone with Derivatives of (-)-Menthol
- PMID: 32856904
- PMCID: PMC7520888
- DOI: 10.1021/acs.jpca.0c06266
Description of Chiral Complexes within Functional-Group Symmetry-Adapted Perturbation Theory-The Case of (S/R)-Carvone with Derivatives of (-)-Menthol
Abstract
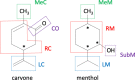
Symmetry-adapted perturbation theory (SAPT) and functional-group SAPT (F-SAPT) are applied to examine differences in interaction energies of diastereoisomeric complexes of two chiral molecules of natural origin: (S/R)-carvone with (-)-menthol. The study is extended by including derivatives of menthol with its hydroxy group exchanged by another functional group, thus examining the substituent effect of the interaction and the interaction differences between diastereoisomers. The partitioning of the interaction energy into functional-group components allows one to explain this phenomenon by the mutual cancellation of attractive and repulsive interactions between functional groups. In some cases, one can identify dominant chiral interactions between groups of atoms of carvone and menthol derivatives, while in many other instances, no major interaction can be distinguished and the net chiral difference results from subtle near cancellation of several smaller terms. Our results indicate that the F-SAPT method can be faithfully utilized for such analyses.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
On the applicability of functional-group symmetry-adapted perturbation theory and other partitioning models for chiral recognition - the case of popular drug molecules interacting with chiral phases.Phys Chem Chem Phys. 2019 Oct 28;21(40):22491-22510. doi: 10.1039/c9cp04056k. Epub 2019 Oct 7. Phys Chem Chem Phys. 2019. PMID: 31588451
-
Chiral Self Recognition: Interactions in Propylene Oxide Complexes.J Phys Chem A. 2019 Oct 10;123(40):8607-8618. doi: 10.1021/acs.jpca.9b06028. Epub 2019 Sep 30. J Phys Chem A. 2019. PMID: 31525971
-
Chemical Assignment of Symmetry-Adapted Perturbation Theory Interaction Energy Components: The Functional-Group SAPT Partition.J Chem Theory Comput. 2014 Oct 14;10(10):4417-31. doi: 10.1021/ct500724p. J Chem Theory Comput. 2014. PMID: 26588139
-
Association Complexes of Calix[6]arenes with Amino Acids Explained by Energy-Partitioning Methods.Molecules. 2022 Nov 16;27(22):7938. doi: 10.3390/molecules27227938. Molecules. 2022. PMID: 36432040 Free PMC article.
-
Analysis of transition state stabilization by non-covalent interactions in the Houk-List model of organocatalyzed intermolecular Aldol additions using functional-group symmetry-adapted perturbation theory.Phys Chem Chem Phys. 2016 Apr 21;18(15):10297-308. doi: 10.1039/c5cp07281f. Epub 2016 Mar 29. Phys Chem Chem Phys. 2016. PMID: 27020417
References
-
- Jeziorski B.; Moszynski R.; Szalewicz K. Perturbation Theory Approach to Intermolecular Potential Energy Surfaces of van der Waals Complexes. Chem. Rev. 1994, 94, 1887–1930. 10.1021/cr00031a008. - DOI
-
- Szalewicz K. Symmetry-Adapted Perturbation Theory of Intermolecular Forces. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 254–272. 10.1002/wcms.86. - DOI
-
- Patkowski K. Recent Developments in Symmetry-Adapted Perturbation Theory. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2020, 10, e145210.1002/wcms.1452. - DOI
LinkOut - more resources
Full Text Sources

