Clinical Characteristics and Viral RNA Detection in Children With Coronavirus Disease 2019 in the Republic of Korea
- PMID: 32857112
- PMCID: PMC7455883
- DOI: 10.1001/jamapediatrics.2020.3988
Clinical Characteristics and Viral RNA Detection in Children With Coronavirus Disease 2019 in the Republic of Korea
Abstract
Importance: There is limited information describing the full spectrum of coronavirus disease 2019 (COVID-19) and the duration of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA detection in children.
Objective: To analyze the full clinical course and the duration of SARS-CoV-2 RNA detectability in children confirmed with COVID-19 in the Republic of Korea, where rigorous public health interventions have been implemented.
Design, setting, and participants: This case series of children with COVID-19 was conducted in 20 hospitals and 2 nonhospital isolation facilities across the country from February 18, 2020, to March 31, 2020. Children younger than 19 years who had COVID-19 were included.
Exposures: Confirmed COVID-19, detected via SARS-CoV-2 RNA in a combined nasopharyngeal and oropharyngeal swab or sputum by real-time reverse transcription-polymerase chain reaction.
Main outcomes and measures: Clinical manifestations during the observation period, including the time and duration of symptom occurrence. The duration of SARS-CoV-2 RNA detection was also analyzed.
Results: A total of 91 children with COVID-19 were included (median [range] age, 11 [0-18] years; 53 boys [58%]). Twenty children (22%) were asymptomatic during the entire observation period. Among 71 symptomatic cases, 47 children (66%) had unrecognized symptoms before diagnosis, 18 (25%) developed symptoms after diagnosis, and only 6 (9%) were diagnosed at the time of symptom onset. Twenty-two children (24%) had lower respiratory tract infections. The mean (SD) duration of the presence of SARS-CoV-2 RNA in upper respiratory samples was 17.6 (6.7) days. Virus RNA was detected for a mean (SD) of 14.1 (7.7) days in asymptomatic individuals. There was no difference in the duration of virus RNA detection between children with upper respiratory tract infections and lower respiratory tract infections (mean [SD], 18.7 [5.8] days vs 19.9 [5.6] days; P = .54). Fourteen children (15%) were treated with lopinavir-ritonavir and/or hydroxychloroquine. All recovered, without any fatal cases.
Conclusions and relevance: In this case series study, inapparent infections in children may have been associated with silent COVID-19 transmission in the community. Heightened surveillance using laboratory screening will allow detection in children with unrecognized SARS-CoV-2 infection.
Conflict of interest statement
Figures
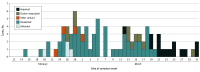
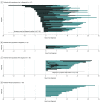

Comment in
-
Symptomatic and Asymptomatic Viral Shedding in Pediatric Patients Infected With Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): Under the Surface.JAMA Pediatr. 2021 Jan 1;175(1):16-18. doi: 10.1001/jamapediatrics.2020.3996. JAMA Pediatr. 2021. PMID: 32857158 No abstract available.
References
-
- World Health Organization Coronavirus disease (COVID-19): situation report–91. Published April 20, 2020. Accessed April 22, 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/2...
-
- Korean Society of Infectious Diseases; Korean Society of Pediatric Infectious Diseases; Korean Society of Epidemiology; Korean Society for Antimicrobial Therapy; Korean Society for Healthcare-associated Infection Control and Prevention; Korea Centers for Disease Control and Prevention . Report on the epidemiological features of coronavirus disease 2019 (COVID-19) outbreak in the Republic of Korea from January 19 to March 2, 2020. J Korean Med Sci. 2020;35(10):e112. doi:10.3346/jkms.2020.35.e112 - DOI - PMC - PubMed
-
- Korea Centers for Disease Control & Prevention COVID-19 response: Korean government’s response system (as of February 25, 2020). Published February 25, 2020. Accessed April 22, 2020. http://ncov.mohw.go.kr/en/baroView.do?brdId=11&brdGubun=111&dataGubun=&n...
-
- Korea Centers for Disease Control & Prevention The updates of COVID-19 in the Republic of Korea. Accessed April 20, 2020. https://www.cdc.go.kr/board/board.es?mid=a20501000000&bid=0015
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

