Assessment of Patient Experiences with Respimat® in Everyday Clinical Practice
- PMID: 32857327
- PMCID: PMC7671953
- DOI: 10.1007/s41030-020-00127-4
Assessment of Patient Experiences with Respimat® in Everyday Clinical Practice
Abstract
Introduction: Chronic obstructive pulmonary disease (COPD) is a progressive disease requiring maintenance therapy. According to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) strategy report, bronchodilation with long-acting muscarinic antagonists (LAMAs) and long-acting β2-agonists (LABAs), administered via inhalers, is currently the mainstay of COPD treatment. Combined LAMA/LABA therapies have been shown to improve patient health status, lung function and breathlessness. Here, we wanted to report patient satisfaction with the Respimat® Soft Mist™ inhaler (SMI).
Methods: This was a pooled analysis of SPIRIT® (NCT02675517) and OTIVACTO® (NCT02719639), two open-label, single-arm, non-interventional studies of physical function in patients with COPD. Patients were treated with tiotropium/olodaterol 5/5 μg for approximately 6 weeks via the SMI. SPIRIT was conducted in Germany; OTIVACTO was conducted in nine European countries. The primary endpoints have been reported previously. Here, we assess patient satisfaction with inhalation and handling, and patient adherence to treatment with the tiotropium/olodaterol SMI in patients with COPD. These were assessed through self-reported questionnaires and physician general assessments.
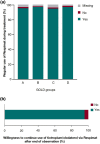
Results: Baseline data were collected from 9180 patients from the SPIRIT and OTIVACTO studies. The majority of patients were GOLD group A (25.59%) or B (46.12%). After 6 weeks of treatment with tiotropium/olodaterol, 85.78% of patients were 'satisfied' or 'very satisfied' with inhaling from the device, and 84.33% of patients were 'satisfied' or 'very satisfied' with the handling of the inhaler. Treating physicians reported patient adherence as 'high' during the study, with 98.57% of patients regularly using the tiotropium/olodaterol SMI. Furthermore, 95.45% of patients expressed a willingness to continue using the tiotropium/olodaterol SMI at the end of the observation period.
Conclusion: In this study, over 9000 patients reported satisfaction with respect to inhalation and handling of the Respimat SMI, and patient adherence was high.
Trial registration: ClinicalTrials.gov: NCT02675517 (SPIRIT) and NCT02719639 (OTIVACTO).
Keywords: Bronchodilator; COPD; Inhaler; Non-interventional studies; OTIVACTO; Observational patient satisfaction; SPIRIT; Soft Mist.
Plain language summary
Inhalation devices are the main method of delivering treatments to patients with chronic obstructive pulmonary disease (COPD). However, there are many devices available, which can lead to confusion and poor inhaler technique. To help doctors decide which device to give to their patients, they consider whether the patient would be happy with the device and whether they can use it correctly. This study pooled data from two large real-life studies to assess patient satisfaction with the Respimat® Soft Mist™ inhaler. Patients assessed their satisfaction and willingness to continue using the device at the end of the study period.The pooled data included over 9000 patients on a range of baseline therapies. After 6 weeks of using the trial device, over 85% of patients were satisfied or very satisfied with inhaling from the device, and over 84% were satisfied with the handling of the device. Physicians reported that nearly 99% of patients regularly used their device. Also, over 95% of the patient population reported that they continued using the inhaler at the end of the study.Overall, these results support the view that many patients with COPD across a wide range of severities and baseline characteristics demonstrated satisfaction with the Respimat® Soft Mist™ inhaler to control their disease.
Figures

Similar articles
-
Assessment of physical functioning and handling of tiotropium/olodaterol Respimat® in patients with COPD in a real-world clinical setting.Int J Chron Obstruct Pulmon Dis. 2019 Jul 4;14:1441-1453. doi: 10.2147/COPD.S195852. eCollection 2019. Int J Chron Obstruct Pulmon Dis. 2019. PMID: 31308649 Free PMC article.
-
An Observational Study Assessing Changes in Health and Functional Status in Patients with Chronic Obstructive Pulmonary Disease (COPD) During Therapy with Spiolto® Respimat® in Everyday Clinical Practice: The Greek ELLACTO Study.Pulm Ther. 2021 Dec;7(2):429-443. doi: 10.1007/s41030-021-00156-7. Epub 2021 May 3. Pulm Ther. 2021. PMID: 33939158 Free PMC article.
-
Impact of tiotropium + olodaterol on physical functioning in COPD: results of an open-label observational study.Int J Chron Obstruct Pulmon Dis. 2016 Apr 27;11:891-8. doi: 10.2147/COPD.S103023. eCollection 2016. Int J Chron Obstruct Pulmon Dis. 2016. PMID: 27217742 Free PMC article.
-
Patient preferences for inhaler devices in chronic obstructive pulmonary disease: experience with Respimat Soft Mist inhaler.Int J Chron Obstruct Pulmon Dis. 2009;4:381-90. doi: 10.2147/copd.s3391. Epub 2009 Oct 19. Int J Chron Obstruct Pulmon Dis. 2009. PMID: 19888356 Free PMC article. Review.
-
Tiotropium Respimat(®) Soft Mist™ inhaler: a review of its use in chronic obstructive pulmonary disease.Drugs. 2014 Oct;74(15):1801-16. doi: 10.1007/s40265-014-0307-4. Drugs. 2014. PMID: 25300412 Review.
Cited by
-
Repurposing mucosal delivery devices for live attenuated tuberculosis vaccines.Front Immunol. 2023 Mar 30;14:1159084. doi: 10.3389/fimmu.2023.1159084. eCollection 2023. Front Immunol. 2023. PMID: 37063870 Free PMC article.
-
Therapeutic Success of Tiotropium/Olodaterol, Measured Using the Clinical COPD Questionnaire (CCQ), in Routine Clinical Practice: A Multinational Non-Interventional Study.Int J Chron Obstruct Pulmon Dis. 2021 Mar 10;16:615-628. doi: 10.2147/COPD.S291920. eCollection 2021. Int J Chron Obstruct Pulmon Dis. 2021. PMID: 33731991 Free PMC article. Clinical Trial.
-
EVELUT®: A Real-World, Observational Study Assessing Dyspnoea and Symptom Burden in COPD Patients Switched from LABA/ICS to LAMA/LABA or LAMA/LABA/ICS.Adv Ther. 2023 Jul;40(7):3263-3278. doi: 10.1007/s12325-023-02524-y. Epub 2023 May 31. Adv Ther. 2023. PMID: 37256536 Free PMC article.
-
An Integrative Transcriptomic and Metabolomic Study Revealed That Melatonin Plays a Protective Role in Chronic Lung Inflammation by Reducing Necroptosis.Front Immunol. 2021 May 4;12:668002. doi: 10.3389/fimmu.2021.668002. eCollection 2021. Front Immunol. 2021. PMID: 34017341 Free PMC article.
References
-
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2020 report). 2019. https://goldcopd.org/wp-content/uploads/2019/11/GOLD-2020-REPORT-ver1.0w.... Cited 15 June 2020.
-
- Miravitlles M, Soler-Cataluña JJ, Alcázar B, Viejo JL, García-Río F. Factors affecting the selection of an inhaler device for COPD and the ideal device for different patient profiles. Results of EPOCA Delphi consensus. Pulmon Pharmacol Ther. 2018;48:97–103. doi: 10.1016/j.pupt.2017.10.006. - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

