Effect of varenicline directly observed therapy versus varenicline self-administered therapy on varenicline adherence and smoking cessation in methadone-maintained smokers: a randomized controlled trial
- PMID: 32857445
- PMCID: PMC7983847
- DOI: 10.1111/add.15240
Effect of varenicline directly observed therapy versus varenicline self-administered therapy on varenicline adherence and smoking cessation in methadone-maintained smokers: a randomized controlled trial
Abstract
Background and aims: Level of adherence to tobacco cessation medication regimens is believed to be causally related to medication effectiveness. This study aimed to evaluate the efficacy of varenicline directly observed therapy (DOT) on varenicline adherence and smoking cessation rates among smokers with opioid use disorder (OUD) receiving methadone treatment.
Design: Multicenter, parallel-group two-arm randomized controlled trial.
Setting: Urban opioid treatment program (OTP) in the Bronx, New York, USA.
Participants: Daily smokers of ≥ 5 cigarettes/day, interested in quitting (ladder of change score 6-8), in methadone treatment for ≥ 3 months, attending OTP ≥ 3 days/week. Participants' mean age was 49 years, 56% were male, 44% Latino, 30% Black, and they smoked a median of 10 cigarettes/day.
Interventions: Individual, block, random assignment to 12 weeks of varenicline, either directly observed with methadone (DOT, n = 50) or via unsupervised self-administered treatment (SAT, n = 50).
Measurements: The primary outcome was adherence measured by pill count. The secondary outcome was 7-day point prevalence tobacco abstinence verified by expired carbon monoxide (CO) < 8 parts per million.
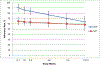
Findings: Retention at 24 weeks was 92%. Mean adherence was 78.5% [95% confidence interval (CI) = 71.8-85.2%] in the DOT group versus 61.8% in the SAT group (95% CI = 55.0-68.6%); differences were driven by DOT effects in the first 6 weeks. CO-verified abstinence did not differ between groups during the intervention (P = 0.26), but was higher in the DOT than the SAT group at intervention end (DOT = 18% versus SAT = 10%, difference = 8%, 95% CI = -13, 28); this difference was not significant (P = 0.39) and was not sustained at 24-week follow-up.
Conclusions: Among daily smokers attending opioid treatment programs, opioid treatment program-based varenicline directly observed therapy was associated with early increases in varenicline adherence compared with self-administered treatment, but findings were inconclusive as to whether directly observed therapy was associated with a difference in tobacco abstinence.
Trial registration: ClinicalTrials.gov NCT01378858.
Keywords: Adherence; directly observed therapy; methadone maintenance; opioid use disorder; smoking cessation; varenicline.
© 2020 Society for the Study of Addiction.
Conflict of interest statement
Declarations of interest:
This project was supported by the National Institutes of Health grant numbers K23DA025736, R25DA023021, R25GM104547, and P30AI051519. Authors receive no direct or indirect funding from the tobacco, alcohol, cannabis, or gaming industries. Dr. Arnsten and Dr. Nahvi receive investigator-initiated grant support from Pfizer. Neither the funding sources nor Pfizer had any role in the study design; data collection, analysis and interpretation; writing the manuscript; or the decision to submit the manuscript for publication.
Figures

References
-
- Stein MD, Weinstock MC, Herman DS, Anderson BJ, Anthony JL, Niaura R. A smoking cessation intervention for the methadone-maintained. Addiction. 2006;101(4):599–607. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

