Saliva or Nasopharyngeal Swab Specimens for Detection of SARS-CoV-2
- PMID: 32857487
- PMCID: PMC7484747
- DOI: 10.1056/NEJMc2016359
Saliva or Nasopharyngeal Swab Specimens for Detection of SARS-CoV-2
Figures
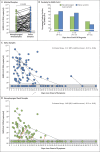
Comment in
-
Saliva sampling for chasing SARS-CoV-2: A Game-changing strategy.Pharmacol Res. 2021 Mar;165:105380. doi: 10.1016/j.phrs.2020.105380. Epub 2020 Dec 16. Pharmacol Res. 2021. PMID: 33338623 Free PMC article. No abstract available.
-
Saliva for Detection of SARS-CoV-2.N Engl J Med. 2021 Mar 4;384(9):e31. doi: 10.1056/NEJMc2032165. Epub 2021 Feb 3. N Engl J Med. 2021. PMID: 33534973 No abstract available.
-
Saliva for Detection of SARS-CoV-2. Reply.N Engl J Med. 2021 Mar 4;384(9):e31. doi: 10.1056/NEJMc2032165. Epub 2021 Feb 3. N Engl J Med. 2021. PMID: 33534974 No abstract available.
References
-
- Kojima N, Turner F, Slepnev V, et al. Self-collected oral fluid and nasal swabs demonstrate comparable sensitivity to clinician collected nasopharyngeal swabs for Covid-19 detection. April 15, 2020. (https://www.medrxiv.org/content/10.1101/2020.04.11.20062372v1). preprint. - PMC - PubMed
-
- Vogels CBF, Brackney D, Wang J, et al. SalivaDirect: simple and sensitive molecular diagnostic test for SARS-CoV-2 surveillance. August 4, 2020 (https://www.medrxiv.org/content/10.1101/2020.08.03.20167791v1). preprint.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
