Photoenzymatic Generation of Unstabilized Alkyl Radicals: An Asymmetric Reductive Cyclization
- PMID: 32857506
- PMCID: PMC7778473
- DOI: 10.1021/jacs.0c07918
Photoenzymatic Generation of Unstabilized Alkyl Radicals: An Asymmetric Reductive Cyclization
Abstract
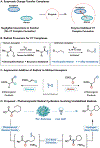
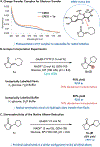
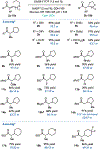
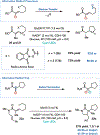
Flavin-dependent "ene"-reductases can generate stabilized alkyl radicals when irradiated with visible light; however, they are not known to form unstabilized radicals. Here, we report an enantioselective radical cyclization using alkyl iodides as precursors to unstabilized nucleophilic radicals. Evidence suggests this species is accessed by photoexcitation of a charge-transfer complex that forms between flavin and substrate within the protein active site. Stereoselective delivery of a hydrogen atom from the flavin semiquinone to the prochiral radical formed after cyclization provides high levels of enantioselectivity across a variety of substrates. Overall, this transformation demonstrates that photoenzymatic catalysis can address long-standing selectivity challenges in the radical literature.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Sibi MP; Manyem S; Zimmerman J Enantioselective Radical Processes. Chem. Rev 2003, 103, 3263–3296. - PubMed
-
- Liang J; Mundorff E; Voladri R; Jenne S; Gilson L; Conway A; Krebber A; Wong J; Huisman G; Truesdell S; Lalonde J Highly Enantioselective Reduction of a Small Heterocycle Ketone: Biocatalytic Reduction of Tetrahedyrothiophene-3-one to the Corresponding (R)-Alcohol. Org. Process Res. Dev 2010, 14, 188–192.
- Chen K; Arnold FH Engineering New Catalytic Activities in Enzymes. Nat. Catal 2020, 3, 203–213.
- Huffman MA et al. Design of an in vitro biocatalytic cascade for the manufacture of islatravir. Science 2019, 366, 1255–1259. - PubMed
- Schober M et al. Chiral Synthesis of LSD1 inhibitor GSK2879552 enabled by direction evolution of an imine reductase. Nat. Catal 2019, 2, 909–915.
-
- Bornscheuer UT; Huisman GW; Kazlauskas RJ; Lutz S; Moore JC; Robins K Engineering the Third Wave of Biocatalysis. Nature 2012, 48, 185. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

