Incidence of intussusception before and after the introduction of rotavirus vaccine in Korea
- PMID: 32857776
- PMCID: PMC7454960
- DOI: 10.1371/journal.pone.0238185
Incidence of intussusception before and after the introduction of rotavirus vaccine in Korea
Abstract
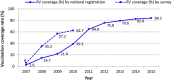
Background: Recent studies have reported that after the introduction of rotavirus vaccine the incidence of intussusception did not change among infants, or slightly increased at the age immediately after the first dose. The rotavirus vaccines were introduced in Korea for private market use in 2007-2008. We investigated the incidence of intussusception before (2002-2006) and after (2009-2015) the vaccine introduction in Korea.
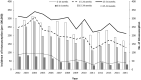
Methods: We conducted an interrupted time series study that used data from the Korean National Health Insurance database to identify infants (<12 months of age) who were diagnosed with intussusception and underwent non-invasive or invasive reduction from 2002 to 2015. According to the recommended ages for immunization, the annual intussusception incidence and the incidence rate ratios were calculated among three age groups, 6-14, 15-24, and 25-34 weeks.
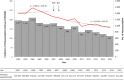
Results: The annual incidences in infants have decreased over time from 241.7 per 100,000 infants (pre-vaccine period) to 160.1-205.2 per 100,000 infants (post-vaccine period). The incidence rate ratio during the post-vaccine period ranged from 0.66 to 0.85. The incidences of intussusception in all three infant age groups have decreased in post-vaccine period compared to pre-vaccine period (incidence rate ratio range: 0.31-0.65, 0.47-0.75, and 0.68-0.94 in 6-14, 15-24, and 25-34 weeks, respectively).
Conclusions: The incidence of intussusception in infants did not increase after the rotavirus vaccine introduction in Korea, but rather decreased over the past decades. Since the incidence of intussusception varies according to country or region, continuous monitoring the incidence of intussusception in infants is necessary in each county or region.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

