Inferences About Drug Safety in Phase III Trials in Oncology: Examples From Advanced Prostate Cancer
- PMID: 32857839
- PMCID: PMC8096372
- DOI: 10.1093/jnci/djaa134
Inferences About Drug Safety in Phase III Trials in Oncology: Examples From Advanced Prostate Cancer
Abstract
Background: Safety is a central consideration when choosing between multiple medications with similar efficacy. We aimed to evaluate whether adverse event (AE) profiles of 3 such drugs in advanced prostate cancer could be distinguished based on published literature.
Methods: We assessed consistency in AE reporting, AE risk in placebo arms, and methodology used for risk estimates and quantification of statistical uncertainty in randomized placebo-controlled phase III trials of apalutamide, enzalutamide, and darolutamide in advanced prostate cancer.
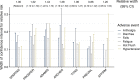
Results: Seven included clinical trials enrolled a total of 9215 participants (range = 1051-1715 per trial) across 3 prostate cancer disease states. Within disease states, baseline patient characteristics appeared similar between trials. Of 54 distinct AE types in total, only 3 (fatigue, hypertension, and seizure) were reported by all 7 trials. Absolute risks of AEs in the placebo arms differed systematically and more than twofold between trials, which was associated with visit frequency and resulted in different degrees of uncertainty in AE profiles between trials. No trial used inferential methodology to quantify statistical uncertainty in AE risks, but 6 of 7 trials drew overall conclusions. Two trials concluded that there was no elevated AE risk because of the intervention, including the trial of darolutamide, which had the greatest statistical uncertainty.
Conclusions: Rigorous comparison of drug safety was precluded by heterogeneity in AE reporting, variation in AE risks in the placebo arms, and lack of inferential statistical methodology, underscoring considerable opportunities to improve how AE data are collected, analyzed, and interpreted in oncology trials.
© The Author(s) 2020. Published by Oxford University Press. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures


References
-
- Levit LA, Perez RP, Smith DC, et al.Streamlining adverse events reporting in oncology: an American Society of Clinical Oncology Research Statement. J Clin Oncol. 2018;36(6):617–623. - PubMed
-
- Trotti A, Colevas AD, Setser A, et al.CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13(3):176–181. - PubMed
-
- Ioannidis JP, Evans SJ, Gotzsche PC, et al.Better reporting of harms in randomized trials: an extension of the CONSORT statement. Ann Intern Med. 2004;141(10):781–788. - PubMed
-
- Sivendran S, Latif A, McBride RB, et al.Adverse event reporting in cancer clinical trial publications. J Clin Oncol. 2014;32(2):83–89. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

