Comparison of plasma etonogestrel concentrations sampled from the contralateral-to-implant and ipsilateral-to-implant arms of contraceptive implant users
- PMID: 32858051
- PMCID: PMC8232040
- DOI: 10.1016/j.contraception.2020.08.011
Comparison of plasma etonogestrel concentrations sampled from the contralateral-to-implant and ipsilateral-to-implant arms of contraceptive implant users
Abstract
Objective: To compare plasma etonogestrel concentrations sampled from the contralateral- versus ipsilateral-to-implant arm.
Study design: Sub-analysis of a cross-sectional study in Botswana in 33 participants who provided contralateral and ipsilateral blood samples.
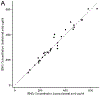
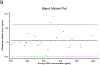
Results: Plasma etonogestrel concentrations in contralateral and ipsilateral specimens were highly correlated (correlation coefficient = 0.99; p < 0.0001). Bland-Altman analysis of agreement showed that etonogestrel levels were on average 5.9 pg/mL higher (2.1%) in ipsilateral compared to contralateral specimens (95% confidence interval: -4.1, 15.9 pg/mL).
Conclusions: We found no meaningful differences in plasma etonogestrel concentrations between samples taken from the contralateral- versus ipsilateral-to-implant arm.
Implications: Our data suggest that etonogestrel plasma concentrations are unlikely to be meaningfully different between samples drawn from the ipsilateral- versus the contralateral-to-implant arms in etonogestrel contraceptive implant users.
Keywords: Contraceptive implant; Contralateral arm; Etonogestrel; Ipsilateral arm; Pharmacokinetics.
Copyright © 2020 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Relationship between patient characteristics and serum etonogestrel concentrations in contraceptive implant users.Contraception. 2019 Jul;100(1):37-41. doi: 10.1016/j.contraception.2019.03.045. Epub 2019 Apr 10. Contraception. 2019. PMID: 30980827 Free PMC article.
-
The influence of lifestyle factors on serum etonogestrel concentrations among contraceptive implant users.Contraception. 2024 Dec;140:110539. doi: 10.1016/j.contraception.2024.110539. Epub 2024 Jul 11. Contraception. 2024. PMID: 39002624
-
The effect of carbamazepine on etonogestrel concentrations in contraceptive implant users.Contraception. 2017 Jun;95(6):571-577. doi: 10.1016/j.contraception.2017.03.004. Epub 2017 Mar 10. Contraception. 2017. PMID: 28288788
-
Medical eligibility criteria for new contraceptive methods: combined hormonal patch, combined hormonal vaginal ring and the etonogestrel implant.Contraception. 2006 Feb;73(2):134-44. doi: 10.1016/j.contraception.2005.08.002. Epub 2005 Oct 20. Contraception. 2006. PMID: 16413844 Review.
-
Best Practices for Counseling Adolescents about the Etonogestrel Implant.J Pediatr Adolesc Gynecol. 2020 Oct;33(5):448-454. doi: 10.1016/j.jpag.2020.06.022. Epub 2020 Jul 2. J Pediatr Adolesc Gynecol. 2020. PMID: 32621879 Review.
References
-
- Croxatto HB, Díaz S, Miranda P, Elamsson K, Johansson EDB. Plasma levels of levonorgestrel in women during longterm use of Norplant. Contraception 1981;23:197–209. - PubMed
-
- Alvarez F, Brache V, Faundes A, Johansson EDB, Odlind V, Nash H. Levonorgestrel plasma levels during continuous administration with different models of subdermal implants. Contraception 1983;27:123–30. - PubMed
-
- Vieira CS, Bahamondes MV, de Souza RM, Brito MB, Rocha Prandini TR, Amaral E, et al. Effect of antiretroviral therapy including lopinavir/ritonavir or efavirenz on etonogestrel-releasing implant pharmacokinetics in HIV-positive women. J Acquir Immune Defic Syndr 2014;66:378–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

