Evaluation of SARS-CoV-2 prototype serologic test in hospitalized patients
- PMID: 32858060
- PMCID: PMC7448777
- DOI: 10.1016/j.clinbiochem.2020.08.008
Evaluation of SARS-CoV-2 prototype serologic test in hospitalized patients
Abstract
Objectives: Humoral immune response to SARS-CoV-2 infection has been reported in several patient cohorts with results that vary by method and population studied due to the lack of reliable commercial assays available as the pandemic initially spread. We sought to clinically assess commercial prototype SARS-CoV-2 IgG and IgA assays for use in screening for prior infection and convalescent plasma donation.
Design and methods: Prototype SARS-CoV-2 IgG and IgA assays from Euroimmun were assessed utilizing remnant specimens. Specificity testing used specimens in their convalescent window for the common coronaviruses and other infectious diseases known to be associated with increased non-specificity in serologic assays. Sensitivity testing utilized serial specimens from molecularly confirmed SARS-CoV-2 critically ill patients to assess seroconversion. Utilizing recombinant spike protein we also developed a competitive confirmation procedure to increase assay specificity.
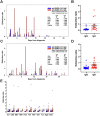
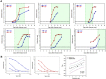
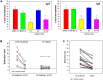
Results: We determined specificity to be 97% and 81%, respectively, when indeterminate samples were considered positive and 99% and 86% when indeterminate samples were considered negative. We developed a new confirmation methodology to enhance the specificity of the assays with an anticipated specificity of 98% for IgA. Valuation of hospitalized COVID-19 patients determined median IgA seroconversion to be 8 days and IgG 10 days. Neither level nor timing of antibody response correlated with days on ventilation. End titer measurements indicate that validated improved assays may be capable of semi-quantitative measurement.
Conclusions: We found these assays to be clinically acceptable for the high prevalence population tested, for instance, for convalescent plasma donation.
Keywords: Antibody; COVID; SARS CoV2; Serology.
Copyright © 2020 The Canadian Society of Clinical Chemists. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- WHO, Laboratory testing for coronavirus disease (COVID-19) in suspected human cases. Interim Guid. (2020).
-
- Lippi G., Simundic A.M., Plebani M. Potential preanalytical and analytical vulnerabilities in the laboratory diagnosis of coronavirus disease 2019 (COVID-19) Clin. Chem. Lab. Med. 2020 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

