Acute brain inflammation, white matter oxidative stress, and myelin deficiency in a model of neonatal intraventricular hemorrhage
- PMID: 32858507
- PMCID: PMC10193502
- DOI: 10.3171/2020.5.PEDS20124
Acute brain inflammation, white matter oxidative stress, and myelin deficiency in a model of neonatal intraventricular hemorrhage
Abstract
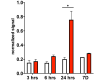
Objective: Neonatal intraventricular hemorrhage (IVH) leads to posthemorrhagic hydrocephalus (PHH), brain injury, and long-term disability. Current therapy for IVH is based on treating PHH but does not address the underlying brain injury. In order to develop pharmacological treatment for IVH, there must be a better understanding of the underlying pathology of this disease. This study was designed to determine the time course of the acute inflammation and oxidative stress that may underlie the progressive pathology of IVH. The authors sought to understand the temporal relationships among inflammation, oxidative stress, and white matter pathology in a rat model of IVH.
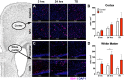
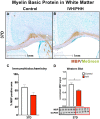
Methods: A rat model of IVH consisting of hemoglobin injection into the lateral ventricle was used. Tissue was analyzed via biochemical and histological methods to map the spatiotemporal distribution of innate immune activation and oxidative stress. White matter was quantified using both immunohistochemistry and Western blot for myelin basic protein (MBP) in the corpus callosum.
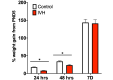
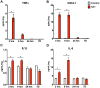
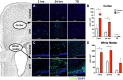
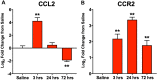
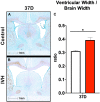
Results: IVH led to acute induction of inflammatory cytokines, followed by oxidative stress. Oxidative stress was concentrated in white matter, adjacent to the lateral ventricles. Animals with IVH initially gained weight at a lower rate than control animals and had larger ventricles and less MBP than control animals.
Conclusions: Experimental IVH induces global inflammation throughout the brain and oxidative stress concentrated in the white matter. Both of these phenomena occur early after IVH. This has implications for human neonates with immature white matter that is exquisitely sensitive to inflammation and oxidative stress. Antiinflammatory or antioxidant therapy for IVH may need to be initiated early in order to protect developing white matter.
Keywords: CCR2; hydrocephalus; intraventricular hemorrhage; microglia.
Conflict of interest statement
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Figures

References
-
- Christian EA, Jin DL, Attenello F, et al. Trends in hospitalization of preterm infants with intraventricular hemorrhage and hydrocephalus in the United States, 2000–2010. J Neurosurg Pediatr. 2016;17(3):260–269. - PubMed
-
- Martin JA, Kung HC, Mathews TJ, et al. Annual summary of vital statistics: 2006. Pediatrics. 2008;121(4):788–801. - PubMed
-
- Mukerji A, Shah V, Shah PS. Periventricular/intraventricular hemorrhage and neurodevelopmental outcomes: a meta-analysis. Pediatrics. 2015;136(6):1132–1143. - PubMed
-
- Kahle KT, Kulkarni AV, Limbrick DD, Jr, Warf BC. Hydrocephalus in children. Lancet. 2016;387(10020):788–799. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

