Allelic variation in rice Fertilization Independent Endosperm 1 contributes to grain width under high night temperature stress
- PMID: 32858766
- PMCID: PMC7756756
- DOI: 10.1111/nph.16897
Allelic variation in rice Fertilization Independent Endosperm 1 contributes to grain width under high night temperature stress
Abstract
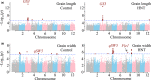
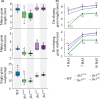
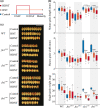
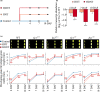
A higher minimum (night-time) temperature is considered a greater limiting factor for reduced rice yield than a similar increase in maximum (daytime) temperature. While the physiological impact of high night temperature (HNT) has been studied, the genetic and molecular basis of HNT stress response remains unexplored. We examined the phenotypic variation for mature grain size (length and width) in a diverse set of rice accessions under HNT stress. Genome-wide association analysis identified several HNT-specific loci regulating grain size as well as loci that are common for optimal and HNT stress conditions. A novel locus contributing to grain width under HNT conditions colocalized with Fie1, a component of the FIS-PRC2 complex. Our results suggest that the allelic difference controlling grain width under HNT is a result of differential transcript-level response of Fie1 in grains developing under HNT stress. We present evidence to support the role of Fie1 in grain size regulation by testing overexpression (OE) and knockout mutants under heat stress. The OE mutants were either unaltered or had a positive impact on mature grain size under HNT, while the knockouts exhibited significant grain size reduction under these conditions.
Keywords: FIS-PRC2; genome-wide association analysis; grain development; grain quality; grain size; heat stress; rice; starch.
© 2020 The Authors New Phytologist © 2020 New Phytologist Trust.
Figures


Similar articles
-
Natural variation in LONELY GUY-Like 1 regulates rice grain weight under warmer night conditions.Plant Physiol. 2024 Sep 2;196(1):164-180. doi: 10.1093/plphys/kiae313. Plant Physiol. 2024. PMID: 38820200 Free PMC article.
-
Genome-wide association study and gene network analyses reveal potential candidate genes for high night temperature tolerance in rice.Sci Rep. 2021 Mar 24;11(1):6747. doi: 10.1038/s41598-021-85921-z. Sci Rep. 2021. PMID: 33762605 Free PMC article.
-
Molecular mapping and characterization of QTLs for grain quality traits in a RIL population of US rice under high nighttime temperature stress.Sci Rep. 2023 Mar 25;13(1):4880. doi: 10.1038/s41598-023-31399-w. Sci Rep. 2023. PMID: 36966148 Free PMC article.
-
High night temperature effects on wheat and rice: Current status and way forward.Plant Cell Environ. 2021 Jul;44(7):2049-2065. doi: 10.1111/pce.14028. Epub 2021 Feb 23. Plant Cell Environ. 2021. PMID: 33576033 Review.
-
High night temperature stress on rice (Oryza sativa) - insights from phenomics to physiology. A review.Funct Plant Biol. 2024 May;51:FP24057. doi: 10.1071/FP24057. Funct Plant Biol. 2024. PMID: 38815128 Review.
Cited by
-
Identification, pyramid, and candidate gene of QTL for yield-related traits based on rice CSSLs in indica Xihui18 background.Mol Breed. 2022 Mar 24;42(4):19. doi: 10.1007/s11032-022-01284-x. eCollection 2022 Apr. Mol Breed. 2022. PMID: 37309460 Free PMC article.
-
Transcriptome enhanced rice grain metabolic model identifies histidine level as a marker for grain chalkiness.Sci Rep. 2025 May 12;15(1):16432. doi: 10.1038/s41598-025-00504-6. Sci Rep. 2025. PMID: 40355482 Free PMC article.
-
Genetic and Molecular Factors Determining Grain Weight in Rice.Front Plant Sci. 2021 Jul 12;12:605799. doi: 10.3389/fpls.2021.605799. eCollection 2021. Front Plant Sci. 2021. PMID: 34322138 Free PMC article. Review.
-
The molecular mechanism by which heat stress during the grain filling period inhibits maize grain filling and reduces yield.Front Plant Sci. 2025 Jan 17;15:1533527. doi: 10.3389/fpls.2024.1533527. eCollection 2024. Front Plant Sci. 2025. PMID: 39898260 Free PMC article.
-
Identifying Heat Adaptability QTLs and Candidate Genes for Grain Appearance Quality at the Flowering Stage in Rice.Rice (N Y). 2025 Mar 11;18(1):13. doi: 10.1186/s12284-025-00770-y. Rice (N Y). 2025. PMID: 40067644 Free PMC article.
References
-
- Abramoff M, Magalhães P, Sunanda RJ. 2004. Image processing with ImageJ. Biophotonics International 11: 36–42.
-
- Ali F, Waters DLE, Ovenden B, Bundock P, Raymond CA, Rose TJ. 2019. Australian rice varieties vary in grain yield response to heat stress during reproductive and grain filling stages. Journal of Agronomy and Crop Science 205: 179–187.
-
- Arshad MS, Farooq M, Asch F, Krishna JSV, Prasad PVV, Siddique KHM. 2017. Thermal stress impacts reproductive development and grain yield in rice. Plant Physiology and Biochemistry 115: 57–72. - PubMed
-
- Bahuguna RN, Solis CA, Shi W, Jagadish KSV. 2017. Post‐flowering night respiration and altered sink activity account for high night temperature‐induced grain yield and quality loss in rice (Oryza sativa L.). Physiologia Plantarum 159: 59–73. - PubMed
-
- Bai X, Zhao H, Huang Y, Xie W, Han Z, Zhang B, Guo Z, Yang L, Dong H, Xue W et al 2016. Genome‐wide association analysis reveals different genetic control in panicle architecture between Indica and Japonica rice. Plant Genome 9: 2. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

