Ethnopharmacology of Fruit Plants: A Literature Review on the Toxicological, Phytochemical, Cultural Aspects, and a Mechanistic Approach to the Pharmacological Effects of Four Widely Used Species
- PMID: 32858815
- PMCID: PMC7504726
- DOI: 10.3390/molecules25173879
Ethnopharmacology of Fruit Plants: A Literature Review on the Toxicological, Phytochemical, Cultural Aspects, and a Mechanistic Approach to the Pharmacological Effects of Four Widely Used Species
Abstract
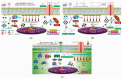
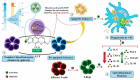
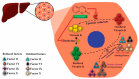
Fruit plants have been widely used by the population as a source of food, income and in the treatment of various diseases due to their nutritional and pharmacological properties. The aim of this study was to review information from the most current research about the phytochemical composition, biological and toxicological properties of four fruit species widely used by the world population in order to support the safe medicinal use of these species and encourage further studies on their therapeutic properties. The reviewed species are: Talisia esculenta, Brosimum gaudichaudii, Genipa americana, and Bromelia antiacantha. The review presents the botanical description of these species, their geographical distribution, forms of use in popular medicine, phytochemical studies and molecules isolated from different plant organs. The description of the pharmacological mechanism of action of secondary metabolites isolated from these species was detailed and toxicity studies related to them were reviewed. The present study demonstrates the significant concentration of phenolic compounds in these species and their anti-inflammatory, anti-tumor, photosensitizing properties, among others. Such species provide important molecules with pharmacological activity that serve as raw materials for the development of new drugs, making further studies necessary to elucidate mechanisms of action not yet understood and prove the safety for use in humans.
Keywords: Bromelia antiacantha; Brosimum gaudichaudii; Genipa americana; Talisia esculenta; biological activity; natural compounds; pharmacological activity; phytochemistry; plant secondary metabolites; plant side effects.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Reis A.F., Schmiele M. Características e potencialidades dos frutos do Cerrado na indústria de alimentos. Brazilian J. Food Technol. 2019;22:e2017150. doi: 10.1590/1981-6723.15017. - DOI
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

