Non-Muscle Myosin II in Axonal Cell Biology: From the Growth Cone to the Axon Initial Segment
- PMID: 32858875
- PMCID: PMC7563147
- DOI: 10.3390/cells9091961
Non-Muscle Myosin II in Axonal Cell Biology: From the Growth Cone to the Axon Initial Segment
Abstract
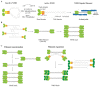
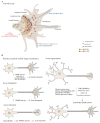
By binding to actin filaments, non-muscle myosin II (NMII) generates actomyosin networks that hold unique contractile properties. Their dynamic nature is essential for neuronal biology including the establishment of polarity, growth cone formation and motility, axon growth during development (and axon regeneration in the adult), radial and longitudinal axonal tension, and synapse formation and function. In this review, we discuss the current knowledge on the spatial distribution and function of the actomyosin cytoskeleton in different axonal compartments. We highlight some of the apparent contradictions and open questions in the field, including the role of NMII in the regulation of axon growth and regeneration, the possibility that NMII structural arrangement along the axon shaft may control both radial and longitudinal contractility, and the mechanism and functional purpose underlying NMII enrichment in the axon initial segment. With the advances in live cell imaging and super resolution microscopy, it is expected that in the near future the spatial distribution of NMII in the axon, and the mechanisms by which it participates in axonal biology will be further untangled.
Keywords: actin ring; actomyosin cytoskeleton; axon growth; axon initial segment; axon regeneration; growth cone; non-muscle myosin II.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

