Lanthanide Luminescence in Visible-Light-Promoted Photochemical Reactions
- PMID: 32858962
- PMCID: PMC7503482
- DOI: 10.3390/molecules25173892
Lanthanide Luminescence in Visible-Light-Promoted Photochemical Reactions
Abstract
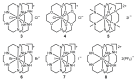
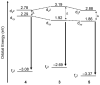
The excitation of lanthanides with visible light to promote photochemical reactions has garnered interest in recent years. Lanthanides serve as initiators for photochemical reactions because they exhibit visible-light-promoted 4f→5d transitions that lead to emissive states with electrochemical potentials that are more negative than the corresponding ground states. The lanthanides that have shown the most promising characteristics for visible-light promoted photoredox are SmII, EuII, and CeIII. By understanding the effects that ligands have on the 5d orbitals of SmII, EuII, and CeIII, luminescence and reactivity can be rationally modulated using coordination chemistry. This review briefly overviews the photochemical reactivity of SmII, EuII, and CeIII with visible light; the properties that influence the reactivity of these ions; and the research that has been reported towards modulating their photochemical-relevant properties using visible light and coordination chemistry.
Keywords: catalysis; lanthanides; luminescence; photoluminescence; photoredox; visible light.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
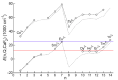
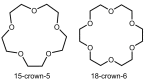
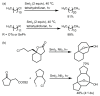
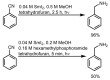
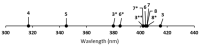
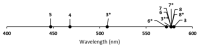

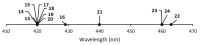

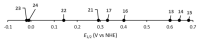
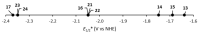
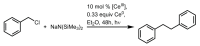
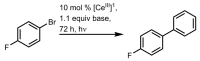
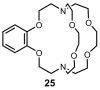
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

