The Effects of Adding Transcutaneous Spinal Cord Stimulation (tSCS) to Sit-To-Stand Training in People with Spinal Cord Injury: A Pilot Study
- PMID: 32858977
- PMCID: PMC7565331
- DOI: 10.3390/jcm9092765
The Effects of Adding Transcutaneous Spinal Cord Stimulation (tSCS) to Sit-To-Stand Training in People with Spinal Cord Injury: A Pilot Study
Abstract
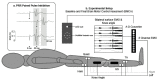
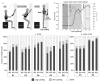
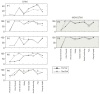
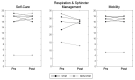
Spinal cord stimulation may enable recovery of volitional motor control in people with chronic Spinal Cord Injury (SCI). In this study we explored the effects of adding SCS, applied transcutaneously (tSCS) at vertebral levels T10/11, to a sit-to-stand training intervention in people with motor complete and incomplete SCI. Nine people with chronic SCI (six motor complete; three motor incomplete) participated in an 8-week intervention, incorporating three training sessions per week. Participants received either tSCS combined with sit-to-stand training (STIM) or sit-to-stand training alone (NON-STIM). Outcome measures were carried out before and after the intervention. Seven participants completed the intervention (STIM N = 5; NON-STIM N = 2). Post training, improvements in International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI) motor scores were noted in three STIM participants (range 1.0-7.0), with no change in NON-STIM participants. Recovery of volitional lower limb muscle activity and/or movement (with tSCS off) was noted in three STIM participants. Unassisted standing was not achieved in any participant, although standing with minimal assistance was achieved in one STIM participant. This pilot study has shown that the recruitment of participants, intervention and outcome measures were all feasible in this study design. However, some modifications are recommended for a larger trial.
Keywords: human; neuromodulation; neurorehabilitation; non-invasive; spinal cord injury; transcutaneous spinal cord stimulation.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures





References
-
- Spinal Cord Injury Centre Physiotherapy Lead Clinicians United Kingdom and Ireland . Clinical Guideline for Standing Adults Following Spinal Cord Injury. MASCIP; Aylesbury, UK: 2013.
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

