An Atomic-Level Perspective of HMG-CoA-Reductase: The Target Enzyme to Treat Hypercholesterolemia
- PMID: 32859023
- PMCID: PMC7503714
- DOI: 10.3390/molecules25173891
An Atomic-Level Perspective of HMG-CoA-Reductase: The Target Enzyme to Treat Hypercholesterolemia
Abstract
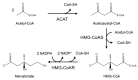
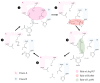
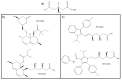
This review provides an updated atomic-level perspective regarding the enzyme 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMG-CoAR), linking the more recent data on this enzyme with a structure/function interpretation. This enzyme catalyzes one of the most important steps in cholesterol biosynthesis and is regarded as one of the most important drug targets in the treatment of hypercholesterolemia. Taking this into consideration, we review in the present article several aspects of this enzyme, including its structure and biochemistry, its catalytic mechanism and different reported and proposed approaches for inhibiting this enzyme, including the commercially available statins or the possibility of using dimerization inhibitors.
Keywords: HMG-CoAR; biochemistry; dimerization inhibitors.; regulation; statins; structure.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Windaus A. Über die konstitution des cholesterins und der gallensäuren. Biol. Chem. 1932;213:147–187. doi: 10.1515/bchm2.1932.213.3-4.147. - DOI
-
- Sheppard A.J., O’Dell R.G., Pennington J.A.T. CHOLESTEROL | Properties and determination. In: Caballero B., editor. Encyclopedia of Food Sciences and Nutrition. 2nd ed. Academic Press; Oxford, UK: 2003. pp. 1220–1226.
-
- Nelson D.L., Lehninger A.L., Cox M.M. Lehninger Principles of Biochemistry. W.H. Freeman; New York, NY, USA: 2008.
-
- Arnold D.R., Kwiterovich P.O. CHOLESTEROL | Absorption, Function, and Metabolism. In: Caballero B., editor. Encyclopedia of Food Sciences and Nutrition. 2nd ed. Academic Press; Oxford, UK: 2003. pp. 1226–1237.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

