Hepatitis B Virus Pre-S Mutants as Biomarkers and Targets for the Development and Recurrence of Hepatocellular Carcinoma
- PMID: 32859114
- PMCID: PMC7552003
- DOI: 10.3390/v12090945
Hepatitis B Virus Pre-S Mutants as Biomarkers and Targets for the Development and Recurrence of Hepatocellular Carcinoma
Abstract
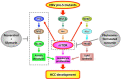
Chronic hepatitis B virus (HBV) infection is a major risk factor for the development of hepatocellular carcinoma (HCC), the leading cause of cancer-related death worldwide. Despite progress in the prevention and therapy of HCC, high incidence and recurrence rates of HCC remain big threats, resulting in poor patient survival. Effective biomarkers and targets of HCC are therefore urgently needed for better management and to improve patient outcomes. Pre-S mutants have been well demonstrated as HBV oncoproteins that play important roles in HCC development through activation of multiple oncogenic signal pathways in hepatocytes, in vitro and in vivo. The presence of pre-S mutants in patients with chronic HBV infection and HBV-related HCC has been associated with a significantly higher risk of HCC development and recurrence after curative surgical resection, respectively. In this review, we summarize the roles of pre-S mutants as biomarkers for predicting HBV-related HCC development and recurrence, and highlight the pre-S mutants-activated oncogenic signal pathways as potential targets for preventing HBV-related HCC development.
Keywords: biomarkers; hepatitis B virus; hepatocellular carcinoma; pre-S mutants; targets.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

