Calcineurin
- PMID: 32859215
- PMCID: PMC7456046
- DOI: 10.1186/s12964-020-00636-4
Calcineurin
Abstract
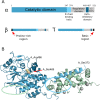
The serine/threonine phosphatase calcineurin acts as a crucial connection between calcium signaling the phosphorylation states of numerous important substrates. These substrates include, but are not limited to, transcription factors, receptors and channels, proteins associated with mitochondria, and proteins associated with microtubules. Calcineurin is activated by increases in intracellular calcium concentrations, a process that requires the calcium sensing protein calmodulin binding to an intrinsically disordered regulatory domain in the phosphatase. Despite having been studied for around four decades, the activation of calcineurin is not fully understood. This review largely focuses on what is known about the activation process and highlights aspects that are currently not understood. Video abstract.
Keywords: Calcineurin; Calcium; Calmodulin; Channels; Intrinsically-disordered region; Microtubules; Mitochondria; Receptors; Transcription.
Conflict of interest statement
The author declares that he has no competing interests.
Figures



References
-
- Klee CB, Ren H, Wang X. Regulation of the calmodulin-stimulated protein phosphatase, calcineurin. J Biol Chem. 1998;273:13367–13370. - PubMed
-
- Rusnak F, Mertz P. Calcineurin: form and function. Physiol Rev. 2000;80:1483–1521. - PubMed
-
- Hogan PG, Li H. Calcineurin. Curr Biol. 2005;15:R442–R443. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

