Bat mammalian orthoreoviruses cause severe pneumonia in mice
- PMID: 32859395
- PMCID: PMC7308043
- DOI: 10.1016/j.virol.2020.05.014
Bat mammalian orthoreoviruses cause severe pneumonia in mice
Abstract
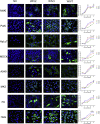
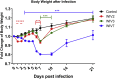
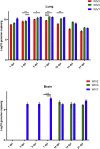
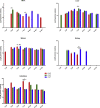
Mammalian orthoreovirus (MRV) infections are ubiquitous in mammals. Increasing evidence suggests that some MRVs can cause severe respiratory disease and encephalitis in humans and other animals. Previously, we isolated six bat MRV strains. However, the pathogenicity of these bat viruses remains unclear. In this study, we investigated the host range and pathogenicity of 3 bat MRV strains (WIV2, 3 and 7) which represent three serotypes. Our results showed that all of them can infect cell lines from different mammalian species and displayed different replication efficiency. The BALB/c mice infected by bat MRVs showed clinical symptoms with systematic infection especially in lung and intestines. Obvious tissue damage were found in all infected lungs. One of the strains, WIV7, showed higher replication efficiency in vitro and vivo and more severe pathogenesis in mice. Our results provide new evidence showing potential pathogenicity of bat MRVs in animals and probable risk in humans.
Keywords: Bat; Mammalian orthoreovirus; Pathogenicity; Pneumonia.
Copyright © 2020 Elsevier Inc. All rights reserved.
Figures




References
-
- Akdis M., Aab A., Altunbulakli C., Azkur K., Costa R.A., Crameri R., Duan S., Eiwegger T., Eljaszewicz A., Ferstl R., Frei R., Garbani M., Globinska A., Hess L., Huitema C., Kubo T., Komlosi Z., Konieczna P., Kovacs N., Kucuksezer U.C., Meyer N., Morita H., Olzhausen J., O'Mahony L., Pezer M., Prati M., Rebane A., Rhyner C., Rinaldi A., Sokolowska M., Stanic B., Sugita K., Treis A., van de Veen W., Wanke K., Wawrzyniak M., Wawrzyniak P., Wirz O.F., Zakzuk J.S., Akdis C.A. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor beta, and TNF-alpha: receptors, functions, and roles in diseases. J. Allergy Clin. Immunol. 2016;138:984–1010. - PubMed
-
- Attoui H., Biagini P., Stirling J., Mertens P.P., Cantaloube J.F., Meyer A., de Micco P., de Lamballerie X. Sequence characterization of Ndelle virus genome segments 1, 5, 7, 8, and 10: evidence for reassignment to the genus Orthoreovirus, family Reoviridae. Biochem. Biophys. Res. Commun. 2001;287:583–588. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

