Platinum Drug Sensitivity Polymorphisms in Stage III Non-small Cell Lung Cancer With Invasion of Mediastinal Lymph Nodes
- PMID: 32859637
- PMCID: PMC7472447
- DOI: 10.21873/cgp.20215
Platinum Drug Sensitivity Polymorphisms in Stage III Non-small Cell Lung Cancer With Invasion of Mediastinal Lymph Nodes
Abstract
Background/aim: Patients with stage IIIA (N2) non-small cell lung cancer (NSCLC) with no progression after induction chemotherapy are usually selected for surgery. Nowadays, response to chemotherapy is not predictable. We aimed to identify genomic predictive markers for response to induction chemotherapy in stage IIIA (N2) NSCLC patients.
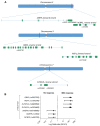
Patients and methods: Whole-exome sequencing (WES) was performed on samples from 11 patients with no response after induction chemotherapy and 6 patients with documented pathological response, admitted to the Hotel Dieu Hospital, Paris or Allegemeines Krakenhaus University, Vienna.
Results: A higher alternative allele frequency was found on SENP5, rs63736860, rs1602 and NCBP2, rs553783 in the non-responder group, and on RGP1, rs1570248, SLFN12L, rs2304968, rs9905892, and GBA2, rs3833700 in the responder group.
Conclusion: These polymorphisms contribute to inter-individual sensibility to chemotherapy response. Interrogation of these genetic variations may have potential applicability when deciding the treatment strategy for patients with stage III NSCLC (N2).
Keywords: NSCLC; Whole-exome sequencing; platinum sensitivity.
Copyright© 2020, International Institute of Anticancer Research (Dr. George J. Delinasios), All rights reserved.
Conflict of interest statement
The Authors declare no conflicts of interest.
Figures



References
-
- Gray JE, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Kurata T, Chiappori A, Lee KH, Cho BC, Planchard D, Paz-Ares L, Faivre-Finn C, Vansteenkiste JF, Spigel DR, Wadsworth C, Taboada M, Dennis PA, Ozguroglu M, Antonia SJ. Three-year overall survival with durvalumab after chemoradiotherapy in stage III NSCLC-update from pacific. J Thorac Oncol. 2020;15(2):288–293. doi: 10.1016/j.jtho.2019.10.002. - DOI - PMC - PubMed
-
- Santana-Davila R, Szabo A, Arce-Lara C, Williams CD, Kelley MJ, Whittle J. Cisplatin versus carboplatin-based regimens for the treatment of patients with metastatic lung cancer. An analysis of veterans health administration data. J Thorac Oncol. 2014;9(5):702–709. doi: 10.1097/JTO.0000000000000146. - DOI - PMC - PubMed
-
- Grigoroiu M, Tagett R, Draghici S, Dima S, Nastase A, Florea R, Sorop A, Ilie V, Bacalbasa N, Tica V, Laszlo V, Mansuet-Lupo A, Damotte D, Klepetko W, Popescu I, Regnard JF. Gene-expression profiling in non-small cell lung cancer with invasion of mediastinal lymph nodes for prognosis evaluation. Cancer Genomics Proteomics. 2015;12(5):231–242. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
