A systematic review of substandard, falsified, unlicensed and unregistered medicine sampling studies: a focus on context, prevalence, and quality
- PMID: 32859648
- PMCID: PMC7454198
- DOI: 10.1136/bmjgh-2020-002393
A systematic review of substandard, falsified, unlicensed and unregistered medicine sampling studies: a focus on context, prevalence, and quality
Abstract
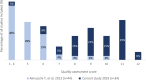
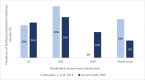
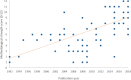
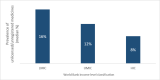
Substandard and falsified (SF) medicines are a global issue contributing to antimicrobial resistance and causing economic and humanitarian harm. To direct law enforcement efficiently, halt the spread of SF medicines and antimicrobial resistance, academics, NGOs and government organisations use medicine quality sampling studies to estimate the prevalence of the problem. A systematic review of medicine quality studies was conducted to estimate how the methodological quality of these studies and SF prevalence has changed between 2013 and 2018. We also aimed to critique medicine sampling study methodologies, and the systematic review process which generates prevalence estimates. Based on 33 studies, the overall estimated median (Q1-Q3) prevalence of SF medicines appears to have remained high at 25% (7.7%-34%) compared with 28.5% in 2013. Furthermore, the methodological quality of prevalence studies has improved over the last 25 years. Definitive conclusions regarding the prevalence of SF medicines cannot be drawn due to the variability in sample sizes, consistency of design methods, and a lack of information concerning contextual factors affecting medicine quality studies. We contend that studies which present cumulative average prevalence figures are useful in a broad sense but could be improved to create more reliable estimates. We propose that medicine quality studies record the context of the study environment to allow systematic reviewers to compare like with like. Although, the academic rigour of medicine quality studies is improving, medicine sampling study limitations still exist. These limitations inhibit the accurate estimation of SF medicine prevalence which is needed to support detailed policy changes.
Keywords: health policy; health services research; health systems evaluation; public health; systematic review.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures



References
-
- WHO A study on the public health and socioeconomic impact of substandard and falsified medical products. [Internet], 2017. Available: http://www.who.int/medicines/regulation/ssffc/publications/Layout-SEstud...
-
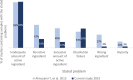
- Grint K, Brookes S, Grint K. Wicked problems and clumsy solutions: the role of leadership : The New Public Leadership Challenge [Internet]. London: Palgrave Macmillan UK, 2010: 169–86.
-
- Naughton B. The future of falsified and substandard medicine detection: digital methods to track and authenticate pharmaceutical products. Pathw Prosper Comm Backgr Pap Ser 2018;5:1–14.
-
- Fake drugs kill more than 250,000 children a year, doctors warn [Internet]. Available: https://www.theguardian.com/science/2019/mar/11/fake-drugs-kill-more-tha...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
