Parvalbumin and Somatostatin Interneurons Contribute to the Generation of Hippocampal Gamma Oscillations
- PMID: 32859716
- PMCID: PMC7531548
- DOI: 10.1523/JNEUROSCI.0261-20.2020
Parvalbumin and Somatostatin Interneurons Contribute to the Generation of Hippocampal Gamma Oscillations
Abstract
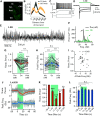
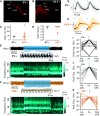
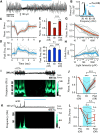
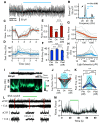
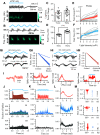
γ-frequency oscillations (30-120 Hz) in cortical networks influence neuronal encoding and information transfer, and are disrupted in multiple brain disorders. While synaptic inhibition is important for synchronization across the γ-frequency range, the role of distinct interneuronal subtypes in slow (<60 Hz) and fast γ states remains unclear. Here, we used optogenetics to examine the involvement of parvalbumin-expressing (PV+) and somatostatin-expressing (SST+) interneurons in γ oscillations in the mouse hippocampal CA3 ex vivo, using animals of either sex. Disrupting either PV+ or SST+ interneuron activity, via either photoinhibition or photoexcitation, led to a decrease in the power of cholinergically induced slow γ oscillations. Furthermore, photoexcitation of SST+ interneurons induced fast γ oscillations, which depended on both synaptic excitation and inhibition. Our findings support a critical role for both PV+ and SST+ interneurons in slow hippocampal γ oscillations, and further suggest that intense activation of SST+ interneurons can enable the CA3 circuit to generate fast γ oscillations.SIGNIFICANCE STATEMENT The generation of hippocampal γ oscillations depends on synchronized inhibition provided by GABAergic interneurons. Parvalbumin-expressing (PV+) interneurons are thought to play the key role in coordinating the spike timing of excitatory pyramidal neurons, but the role distinct inhibitory circuits in network synchronization remains unresolved. Here, we show, for the first time, that causal disruption of either PV+ or somatostatin-expressing (SST+) interneuron activity impairs the generation of slow γ oscillations in the ventral hippocampus ex vivo We further show that SST+ interneuron activation along with general network excitation is sufficient to generate high-frequency γ oscillations in the same preparation. These results affirm a crucial role for both PV+ and SST+ interneurons in hippocampal γ oscillation generation.
Keywords: gamma; hippocampus; interneuron; oscillation; parvalbumin; somatostatin.
Copyright © 2020 the authors.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
