Prefrontal Cortex-Driven Dopamine Signals in the Striatum Show Unique Spatial and Pharmacological Properties
- PMID: 32859717
- PMCID: PMC7511190
- DOI: 10.1523/JNEUROSCI.1327-20.2020
Prefrontal Cortex-Driven Dopamine Signals in the Striatum Show Unique Spatial and Pharmacological Properties
Abstract
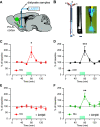
Dopamine (DA) signals in the striatum are critical for a variety of vital processes, including motivation, motor learning, and reinforcement learning. Striatal DA signals can be evoked by direct activation of inputs from midbrain DA neurons (DANs) as well as cortical and thalamic inputs to the striatum. In this study, we show that in vivo optogenetic stimulation of prelimbic (PrL) and infralimbic (IL) cortical afferents to the striatum triggers an increase in extracellular DA concentration, which coincides with elevation of striatal acetylcholine (ACh) levels. This increase is blocked by a nicotinic ACh receptor (nAChR) antagonist. Using single or dual optogenetic stimulation in brain slices from male and female mice, we compared the properties of these PrL/IL-evoked DA signals with those evoked by stimulation from midbrain DAN axonal projections. PrL/IL-evoked DA signals are undistinguishable from DAN evoked DA signals in their amplitudes and electrochemical properties. However, PrL/IL-evoked DA signals are spatially restricted and preferentially recorded in the dorsomedial striatum. PrL/IL-evoked DA signals also differ in their pharmacological properties, requiring activation of glutamate and nicotinic ACh receptors. Thus, both in vivo and in vitro results indicate that cortical evoked DA signals rely on recruitment of cholinergic interneurons, which renders DA signals less able to summate during trains of stimulation and more sensitive to both cholinergic drugs and temperature. In conclusion, cortical and midbrain inputs to the striatum evoke DA signals with unique spatial and pharmacological properties that likely shape their functional roles and behavioral relevance.SIGNIFICANCE STATEMENT Dopamine signals in the striatum play a critical role in basal ganglia function, such as reinforcement and motor learning. Different afferents to the striatum can trigger dopamine signals, but their release properties are not well understood. Further, these input-specific dopamine signals have only been studied in separate animals. Here we show that optogenetic stimulation of cortical glutamatergic afferents to the striatum triggers dopamine signals both in vivo and in vitro These afferents engage cholinergic interneurons, which drive dopamine release from dopamine neuron axons by activation of nicotinic acetylcholine receptors. We also show that cortically evoked dopamine signals have other unique properties, including spatial restriction and sensitivity to temperature changes than dopamine signals evoked by stimulation of midbrain dopamine neuron axons.
Keywords: DA release; PFC; dorsomedial striatum; fast-scan cyclic voltammetry; optogenetics.
Copyright © 2020 the authors.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
