Sex differences in oncogenic mutational processes
- PMID: 32859912
- PMCID: PMC7455744
- DOI: 10.1038/s41467-020-17359-2
Sex differences in oncogenic mutational processes
Abstract
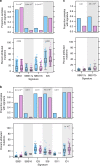
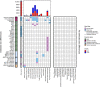
Sex differences have been observed in multiple facets of cancer epidemiology, treatment and biology, and in most cancers outside the sex organs. Efforts to link these clinical differences to specific molecular features have focused on somatic mutations within the coding regions of the genome. Here we report a pan-cancer analysis of sex differences in whole genomes of 1983 tumours of 28 subtypes as part of the ICGC/TCGA Pan-Cancer Analysis of Whole Genomes (PCAWG) Consortium. We both confirm the results of exome studies, and also uncover previously undescribed sex differences. These include sex-biases in coding and non-coding cancer drivers, mutation prevalence and strikingly, in mutational signatures related to underlying mutational processes. These results underline the pervasiveness of molecular sex differences and strengthen the call for increased consideration of sex in molecular cancer research.
Conflict of interest statement
The authors declare no competing interests.
Figures


Comment in
-
Sexual dimorphism in cancer.Nat Rev Cancer. 2020 Nov;20(11):627. doi: 10.1038/s41568-020-00304-2. Nat Rev Cancer. 2020. PMID: 32918061 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
- 25813/CRUK_/Cancer Research UK/United Kingdom
- R01 CA235162/CA/NCI NIH HHS/United States
- MC_UU_00016/11/MRC_/Medical Research Council/United Kingdom
- MR/L008963/1/MRC_/Medical Research Council/United Kingdom
- HHMI/Howard Hughes Medical Institute/United States
- T32 HG002295/HG/NHGRI NIH HHS/United States
- MC_UU_12009/11/MRC_/Medical Research Council/United Kingdom
- P30 CA016042/CA/NCI NIH HHS/United States
- 27176/CRUK_/Cancer Research UK/United Kingdom
- MC_U137961146/MRC_/Medical Research Council/United Kingdom
- P50 CA211015/CA/NCI NIH HHS/United States
- T32 GM008313/GM/NIGMS NIH HHS/United States
- U24 CA210969/CA/NCI NIH HHS/United States
- 29567/CRUK_/Cancer Research UK/United Kingdom
- G1000729/MRC_/Medical Research Council/United Kingdom
- U01 CA214194/CA/NCI NIH HHS/United States
- R35 GM127029/GM/NIGMS NIH HHS/United States
- P30 ES010126/ES/NIEHS NIH HHS/United States
- R01 CA183793/CA/NCI NIH HHS/United States
- 26718/CRUK_/Cancer Research UK/United Kingdom
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

