Osteoclast-associated receptor blockade prevents articular cartilage destruction via chondrocyte apoptosis regulation
- PMID: 32859940
- PMCID: PMC7455568
- DOI: 10.1038/s41467-020-18208-y
Osteoclast-associated receptor blockade prevents articular cartilage destruction via chondrocyte apoptosis regulation
Abstract
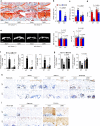
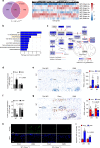
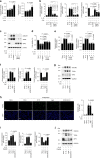
Osteoarthritis (OA), primarily characterized by articular cartilage destruction, is the most common form of age-related degenerative whole-joint disease. No disease-modifying treatments for OA are currently available. Although OA is primarily characterized by cartilage destruction, our understanding of the processes controlling OA progression is poor. Here, we report the association of OA with increased levels of osteoclast-associated receptor (OSCAR), an immunoglobulin-like collagen-recognition receptor. In mice, OSCAR deletion abrogates OA manifestations, such as articular cartilage destruction, subchondral bone sclerosis, and hyaline cartilage loss. These effects are a result of decreased chondrocyte apoptosis, which is caused by the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in induced OA. Treatments with human OSCAR-Fc fusion protein attenuates OA pathogenesis caused by experimental OA. Thus, this work highlights the function of OSCAR as a catabolic regulator of OA pathogenesis, indicating that OSCAR blockade is a potential therapy for OA.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Molecular regulation of articular chondrocyte function and its significance in osteoarthritis.Histol Histopathol. 2011 Mar;26(3):377-94. doi: 10.14670/HH-26.377. Histol Histopathol. 2011. PMID: 21210351 Review.
-
Sotrastaurin, a PKC inhibitor, attenuates RANKL-induced bone resorption and attenuates osteochondral pathologies associated with the development of OA.J Cell Mol Med. 2020 Aug;24(15):8452-8465. doi: 10.1111/jcmm.15404. Epub 2020 Jul 11. J Cell Mol Med. 2020. PMID: 32652826 Free PMC article.
-
Rebamipide Attenuates Mandibular Condylar Degeneration in a Murine Model of TMJ-OA by Mediating a Chondroprotective Effect and by Downregulating RANKL-Mediated Osteoclastogenesis.PLoS One. 2016 Apr 28;11(4):e0154107. doi: 10.1371/journal.pone.0154107. eCollection 2016. PLoS One. 2016. PMID: 27123995 Free PMC article.
-
Chondrocyte Apoptosis in the Pathogenesis of Osteoarthritis.Int J Mol Sci. 2015 Oct 30;16(11):26035-54. doi: 10.3390/ijms161125943. Int J Mol Sci. 2015. PMID: 26528972 Free PMC article. Review.
-
Aliskiren has chondroprotective efficacy in a rat model of osteoarthritis through suppression of the local renin-angiotensin system.Mol Med Rep. 2017 Oct;16(4):3965-3973. doi: 10.3892/mmr.2017.7110. Epub 2017 Jul 28. Mol Med Rep. 2017. PMID: 28765966 Free PMC article.
Cited by
-
A nanozyme-functionalized bilayer hydrogel scaffold for modulating the inflammatory microenvironment to promote osteochondral regeneration.J Nanobiotechnology. 2024 Jul 28;22(1):445. doi: 10.1186/s12951-024-02723-x. J Nanobiotechnology. 2024. PMID: 39069607 Free PMC article.
-
Polyherbal formulation PL02 alleviates pain, inflammation, and subchondral bone deterioration in an osteoarthritis rodent model.Front Nutr. 2023 Nov 16;10:1217051. doi: 10.3389/fnut.2023.1217051. eCollection 2023. Front Nutr. 2023. PMID: 38045809 Free PMC article.
-
BCLAF1 Regulates Osteoarthritic Cartilage Degradation Through Interaction with LAMTOR2.Int J Biol Sci. 2025 Feb 3;21(4):1666-1685. doi: 10.7150/ijbs.100396. eCollection 2025. Int J Biol Sci. 2025. PMID: 39990659 Free PMC article.
-
The role of TNFRSF11B in development of osteoarthritic cartilage.Rheumatology (Oxford). 2022 Feb 2;61(2):856-864. doi: 10.1093/rheumatology/keab440. Rheumatology (Oxford). 2022. PMID: 33989379 Free PMC article.
-
Identification of SLC3A2 as a Potential Therapeutic Target of Osteoarthritis Involved in Ferroptosis by Integrating Bioinformatics, Clinical Factors and Experiments.Cells. 2022 Oct 30;11(21):3430. doi: 10.3390/cells11213430. Cells. 2022. PMID: 36359826 Free PMC article.
References
-
- Hunter DJ, Schofield D, Callander E. The individual and socioeconomic impact of osteoarthritis. Nat. Rev. Rheumatol. 2014;10:437–441. - PubMed
-
- Lories RJ, Luyten F. The bone-cartilage unit in osteoarthritis. Nat. Rev. Rheumatol. 2011;7:43–49. - PubMed
-
- Heinemeier KM, et al. Radiocarbon dating reveals minimal collagen turnover in both healthy and osteoarthritic human cartilage. Sci. Transl. Med. 2016;8:346ra390. - PubMed
-
- Latourte A, et al. Systemic inhibition of IL-6/Stat3 signalling protects against experimental osteoarthritis. Ann. Rheum. Dis. 2017;76:748–755. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

