Mitigating the risk of cytokine release syndrome in a Phase I trial of CD20/CD3 bispecific antibody mosunetuzumab in NHL: impact of translational system modeling
- PMID: 32859946
- PMCID: PMC7455723
- DOI: 10.1038/s41540-020-00145-7
Mitigating the risk of cytokine release syndrome in a Phase I trial of CD20/CD3 bispecific antibody mosunetuzumab in NHL: impact of translational system modeling
Abstract
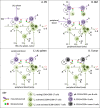
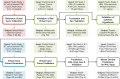
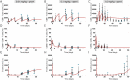
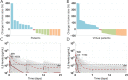
Mosunetuzumab, a T-cell dependent bispecific antibody that binds CD3 and CD20 to drive T-cell mediated B-cell killing, is currently being tested in non-Hodgkin lymphoma. However, potent immune stimulation with T-cell directed therapies poses the risk of cytokine release syndrome, potentially limiting dose and utility. To understand mechanisms behind safety and efficacy and explore safety mitigation strategies, we developed a novel mechanistic model of immune and antitumor responses to the T-cell bispecifics (mosunetuzumab and blinatumomab), including the dynamics of B- and T-lymphocytes in circulation, lymphoid tissues, and tumor. The model was developed and validated using mosunetuzumab nonclinical and blinatumomab clinical data. Simulations delineated mechanisms contributing to observed cell and cytokine (IL6) dynamics and predicted that initial step-fractionated dosing limits systemic T-cell activation and cytokine release without compromising tumor response. These results supported a change to a step-fractionated treatment schedule of mosunetuzumab in the ongoing Phase I clinical trial, enabling safer administration of higher doses.
Conflict of interest statement
I.H., K.G., E.S., C.-C.L., and S.R. are all employees of Genentech Inc.; L.L.S. was an employee of Genentech at the time the studies were conducted and is currently an employee at Harpoon Therapeutics. Y.-W.C. was an employee of Genentech at the time the studies were conducted and is currently an employee at Fate Therapeutics. Genentech has filed patent applications related to TDBs, and is currently conducting clinical studies of the molecule mosunetuzumab. Genentech holds no stake in the molecule blinatumomab, which is a clinically-approved molecule for the treatment of ALL. Investigators may request materials from Genentech by submitting a request form at
Figures

References
-
- Hiddemann W, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005;106:3725–3732. doi: 10.1182/blood-2005-01-0016. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

