β-Catenin induces transcriptional expression of PD-L1 to promote glioblastoma immune evasion
- PMID: 32860047
- PMCID: PMC7596815
- DOI: 10.1084/jem.20191115
β-Catenin induces transcriptional expression of PD-L1 to promote glioblastoma immune evasion
Abstract
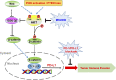
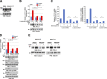
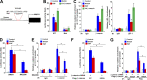
PD-L1 up-regulation in cancer contributes to immune evasion by tumor cells. Here, we show that Wnt ligand and activated EGFR induce the binding of the β-catenin/TCF/LEF complex to the CD274 gene promoter region to induce PD-L1 expression, in which AKT activation plays an important role. β-Catenin depletion, AKT inhibition, or PTEN expression reduces PD-L1 expression in tumor cells, enhances activation and tumor infiltration of CD8+ T cells, and reduces tumor growth, accompanied by prolonged mouse survival. Combined treatment with a clinically available AKT inhibitor and an anti-PD-1 antibody overcomes tumor immune evasion and greatly inhibits tumor growth. In addition, AKT-mediated β-catenin S552 phosphorylation and nuclear β-catenin are positively correlated with PD-L1 expression and inversely correlated with the tumor infiltration of CD8+ T cells in human glioblastoma specimens, highlighting the clinical significance of β-catenin activation in tumor immune evasion.
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures








Comment in
-
β-Catenin regulates tumor-derived PD-L1.J Exp Med. 2020 Nov 2;217(11):e20200684. doi: 10.1084/jem.20200684. J Exp Med. 2020. PMID: 32860048 Free PMC article.
References
-
- Cabodevilla A.G., Sánchez-Caballero L., Nintou E., Boiadjieva V.G., Picatoste F., Gubern A., and Claro E.. 2013. Cell survival during complete nutrient deprivation depends on lipid droplet-fueled β-oxidation of fatty acids. J. Biol. Chem. 288:27777–27788. 10.1074/jbc.M113.466656 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

