Model-Based Meta-Analysis Compares DAS28 Rheumatoid Arthritis Treatment Effects and Suggests an Expedited Trial Design for Early Clinical Development
- PMID: 32860421
- PMCID: PMC7894503
- DOI: 10.1002/cpt.2023
Model-Based Meta-Analysis Compares DAS28 Rheumatoid Arthritis Treatment Effects and Suggests an Expedited Trial Design for Early Clinical Development
Abstract
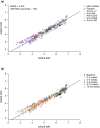
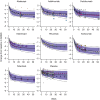
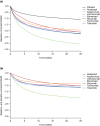
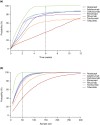
A nonlinear mixed effects modeling approach was used to conduct a model-based meta-analysis (MBMA) of longitudinal, summary-level, baseline-corrected 28-joint Disease Activity Score (ΔDAS28) clinical trial data from seven approved rheumatoid arthritis (RA) drugs (abatacept, adalimumab, certolizumab, etanercept, rituximab, tocilizumab, and tofacitinib), representing 130 randomized clinical trials in 27,355 patients. All of the drugs except tocilizumab were found to have relatively similar ΔDAS28 time courses and efficacy (baseline-corrected and placebo-corrected) at 24 weeks and beyond of approximately 0.87-1.3 units in the typical RA patient population. Tocilizumab was estimated to have a differentially greater response of 1.99 at 24 weeks, likely due to its disproportionate effect on the acute-phase cytokine interleukin-6. Baseline DAS28, disease duration, percentage of male participants, and the year of conduct of the trial were found to have statistically significant effects on the timing and/or magnitude of ΔDAS28 in the control arms. Clinical trial simulations using the present MBMA indicated that abatacept, certolizumab, etanercept, tocilizumab, and tofacitinib would be expected to have a greater than 70% probability of showing a statistically significant difference vs. control at Week 6 with a sample size of ~ 30 patients per arm. In future RA clinical trials, an interim analysis conducted as early as 6 weeks after treatment initiation, with relatively small sample sizes, should be sufficient to detect the ΔDAS28 treatment effect vs. placebo.
© 2020 Bristol-Myers Squibb. Clinical Pharmacology & Therapeutics published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
All authors are employees of Bristol Myers Squibb and hold company stock.
Figures
Similar articles
-
[The efficacy and safety of tocilizumab combined with disease-modifying anti-rheumatoid drugs in the treatment of active rheumatoid arthritis: a multi-center, randomized, double-blinded, placebo-controlled trial].Zhonghua Nei Ke Za Zhi. 2013 Apr;52(4):323-9. Zhonghua Nei Ke Za Zhi. 2013. PMID: 23925361 Clinical Trial. Chinese.
-
Cost per responder associated with biologic therapies for Crohn's disease, psoriasis, and rheumatoid arthritis.Adv Ther. 2012 Jul;29(7):620-34. doi: 10.1007/s12325-012-0035-7. Epub 2012 Jul 27. Adv Ther. 2012. PMID: 22843208
-
Early rheumatoid arthritis treated with tocilizumab, methotrexate, or their combination (U-Act-Early): a multicentre, randomised, double-blind, double-dummy, strategy trial.Lancet. 2016 Jul 23;388(10042):343-355. doi: 10.1016/S0140-6736(16)30363-4. Epub 2016 Jun 7. Lancet. 2016. PMID: 27287832 Clinical Trial.
-
Sarilumab for Previously-Treated Moderate or Severe Rheumatoid Arthritis: An Evidence Review Group Perspective of a NICE Single Technology Appraisal.Pharmacoeconomics. 2018 Dec;36(12):1427-1437. doi: 10.1007/s40273-018-0677-7. Pharmacoeconomics. 2018. PMID: 29882210 Review.
-
Rheumatoid arthritis patients treated in trial and real world settings: comparison of randomized trials with registries.Rheumatology (Oxford). 2018 Feb 1;57(2):354-369. doi: 10.1093/rheumatology/kex394. Rheumatology (Oxford). 2018. PMID: 29149289 Review.
Cited by
-
Pharmacometrics meets statistics-A synergy for modern drug development.CPT Pharmacometrics Syst Pharmacol. 2021 Oct;10(10):1134-1149. doi: 10.1002/psp4.12696. Epub 2021 Aug 19. CPT Pharmacometrics Syst Pharmacol. 2021. PMID: 34318621 Free PMC article.
-
Applications of Model-Based Meta-Analysis in Drug Development.Pharm Res. 2022 Aug;39(8):1761-1777. doi: 10.1007/s11095-022-03201-5. Epub 2022 Feb 16. Pharm Res. 2022. PMID: 35174432 Free PMC article.
-
Monotreatment With Conventional Antirheumatic Drugs or Glucocorticoids in Rheumatoid Arthritis: A Network Meta-Analysis.JAMA Netw Open. 2023 Oct 2;6(10):e2335950. doi: 10.1001/jamanetworkopen.2023.35950. JAMA Netw Open. 2023. PMID: 37801318 Free PMC article.
-
Joint longitudinal model-based meta-analysis of FEV1 and exacerbation rate in randomized COPD trials.J Pharmacokinet Pharmacodyn. 2023 Aug;50(4):297-314. doi: 10.1007/s10928-023-09853-z. Epub 2023 Mar 22. J Pharmacokinet Pharmacodyn. 2023. PMID: 36947282 Free PMC article.
-
Impact of vitamin D supplementation on disease activity and pain management in rheumatoid arthritis: a randomized double-blinded controlled study.BMC Rheumatol. 2025 Jul 11;9(1):87. doi: 10.1186/s41927-025-00543-6. BMC Rheumatol. 2025. PMID: 40646666 Free PMC article.
References
-
- McInnes, I.B. & Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 365, 2205–2219 (2011). - PubMed
-
- Prevoo, M.L. , van't Hof, M.A. , Kuper, H.H. , van Leeuwen, M.A. , van de Putte, L.B. & van Riel, P.L. Modified disease activity scores that include twenty‐eight‐joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 38, 44–48 (1995). - PubMed
-
- Fransen, J. & van Riel, P.L.C.M. The disease activity score and the EULAR response criteria. Rheum. Dis. Clin. North. Am. 35, 745–757, vii‐viii (2009). - PubMed
-
- van der Heijde, D.M. , van 't Hof, M. , van Riel, P.L. , & van de Putte, L.B. Development of a disease activity score based on judgment in clinical practice by rheumatologists. J. Rheumatol. 20, 579–581 (1993). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

