Benefit-Risk Assessment of Esketamine Nasal Spray vs. Placebo in Treatment-Resistant Depression
- PMID: 32860422
- PMCID: PMC7894501
- DOI: 10.1002/cpt.2024
Benefit-Risk Assessment of Esketamine Nasal Spray vs. Placebo in Treatment-Resistant Depression
Abstract
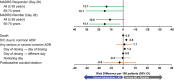
This post hoc analysis assessed the benefit-risk profile of esketamine nasal spray + oral antidepressant (AD) induction and maintenance treatment in patients with treatment-resistant depression (TRD). The Benefit-Risk Action Team framework was utilized to assess the benefit-risk profile using data from three induction studies and one maintenance study. Benefits were proportion of remitters or responders in induction studies and proportion of stable remitters or stable responders who remained relapse-free in the maintenance study. Risks were death, suicidal ideation, most common adverse events (AEs), and potential long-term risks. Per 100 patients on esketamine + AD vs. AD + placebo in induction therapy, 5-21 additional patients would remit and 14-17 additional patients would respond. In maintenance therapy, 19-32 fewer relapses would occur with esketamine. In both cases, there was little difference in serious or severe common AEs (primarily dissociation, vertigo, and dizziness). These findings support a positive benefit-risk balance for esketamine + AD as induction and maintenance treatment in patients with TRD.
Trial registration: ClinicalTrials.gov NCT02417064 NCT02418585 NCT02422186 NCT02493868.
© 2020 Janssen. Clinical Pharmacology & Therapeutics published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
All authors are employees of Janssen Research & Development, LLC.
Figures



References
-
- US Food and Drug Administration . Structured approach to benefit‐risk assessment in drug regulatory decision‐making <http://www.fda.gov/downloads/ForIndustry/UserFees/PrescriptionDrugUserFe...> (2019). Accessed November 15, 2019.
-
- Luteijn, J.M. et al State of the art in benefit‐risk analysis: medicines. Food Chem. Toxicol. 50, 26–32 (2012). - PubMed
-
- Pignatti, F. et al Structured frameworks to increase the transparency of the assessment of benefits and risks of medicines: current status and possible future directions. Clin. Pharmacol. Ther. 98, 522–533 (2015). - PubMed
-
- ICH harmonised guideline: revision of M4E guideline on enhancing the format and structure of benefit‐risk information in ICH, efficacy ‐ M4E(R2) <https://database.ich.org/sites/default/files/M4E_R2__Guideline.pdf> (2016). Accessed March 12, 2020.
-
- Levitan, B. A concise display of multiple end points for benefit‐risk assessment. Clin. Pharmacol. Ther. 89, 56–59 (2011). - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

